Epigenetics, which governs whether specific genes in the body are turned on or not, has broad effects on health and development, ranging from the propensity to develop cancer to a disposition to become fat or thin. That has made epigenetic inheritance—the idea that these patterns of gene expression can be passed from parents to children, grandchildren, and beyond, the subject of profuse research. Some investigators have begun to treat it as settled science. But Karin Michels, Sc.D. ’95, brought bracing skepticism to the question of whether epigenetic information in mammals can be transferred across generations during a talk earlier this year at the Radcliffe Institute, where she has been a fellow.
Every cell in a human body has the same DNA, or underlying genetic code, explained Michels, who chairs the department of epidemiology at UCLA’s Fielding School of Public Health. Epigenetics governs how those genes are expressed at every stage of life. During development, for example, epigenetic markers govern the differentiation that makes a muscle cell different from a kidney cell purely through the genes that are activated—and then maintains that program from one generation of cell to the next, so muscle remains muscle, and kidney remains kidney. In a monarch butterfly, the caterpillar, cocoon, and winged stages of its lifecycle—all different expressions, or phenotypes, of the same underlying DNA—are also under epigenetic control. But what is distinctive about epigenetic switches is that they can change. Diet, psychological state, exposure to cigarette smoke, exercise, financial status: a whole range of environmental or lifestyle factors can modulate gene expression, turning genes on or off.
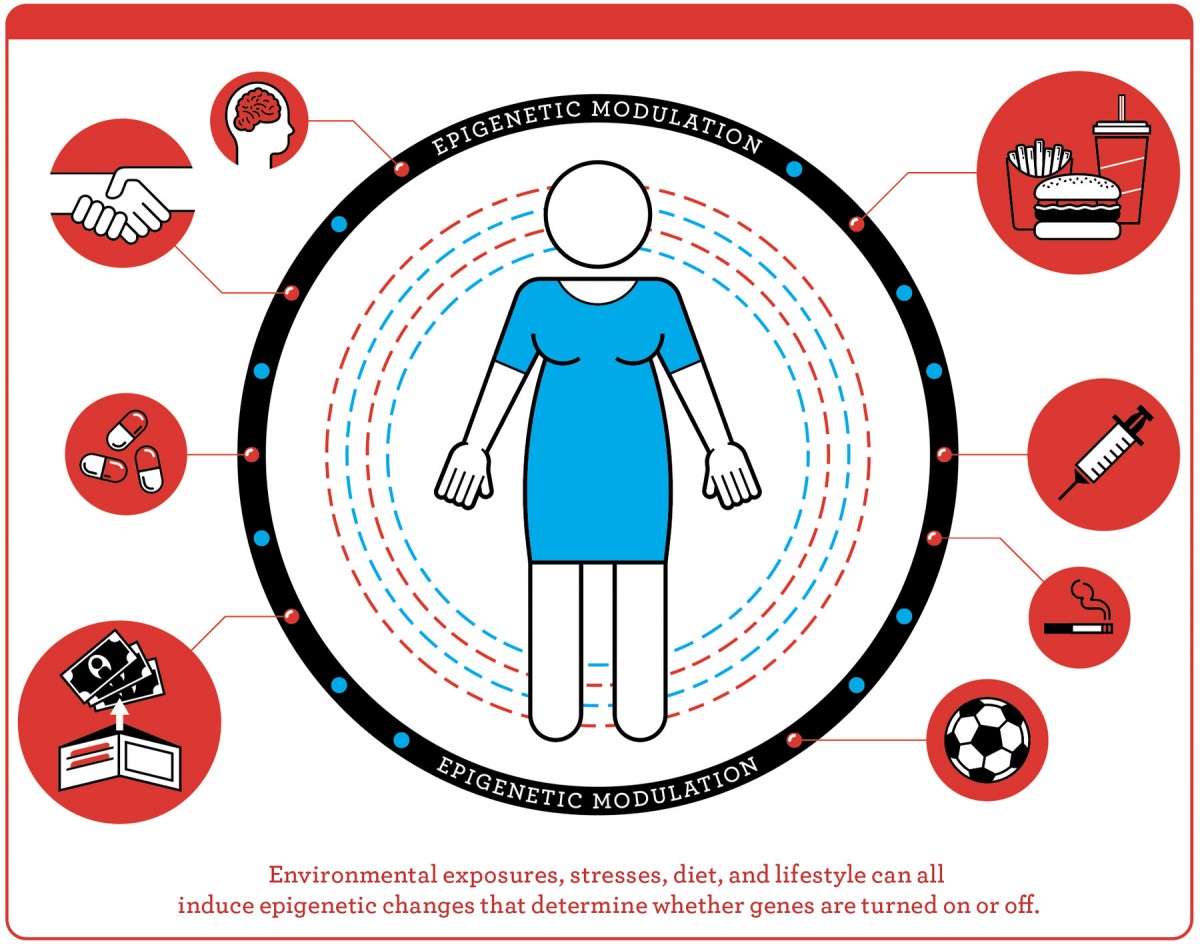 Illustration by Jude Buffum
Illustration by Jude BuffumResearch published recently in scientific journals such as Cell, Nature, and Nature Genetics has suggested that epigenetic information can be passed from one generation to the next. But that may be incorrect. A grandmother who smokes, thus altering her own epigenome, could in theory pass on the harmful epigenetic configuration caused by her habit. Research has shown that smoking can cause abnormal increases in hormones that signal hunger, and if this is heritable, that could lead to obesity in her granddaughter.
But has science proven that these are transgenerational effects? Far from it, said Michels. First, she pointed out, evolution militates against epigenetic inheritance. Epigenetic changes take place through three different mechanisms, the best studied of which is DNA methylation. Methyl groups, a methane-derived group of atoms that are layered on top of DNA molecules, provide instructions for which genes should be turned on or off—but during reproduction, mammalian cells go through two full cycles of demethylation. That process strips all methyl groups, and thus epigenetic information, from germ and embryonic cells. To date, there is no evidence that epigenetic information can survive two rounds of this biochemical cleansing.
Second, proving that an epigenetic configuration can be passed transgenerationally would require ruling out the possibility that any observed effect might have resulted from exposure in the womb. Michels, an epigenetic epidemiologist, studies such exposures and cited one famous experimental example in mice. A pregnant yellow-furred Agouti fed a diet rich in methyl donor groups (a stand-in for a healthy diet), has predominantly healthy offspring with brown coats. But if fed regular mouse food, the mother gives birth mainly to obese, yellow-furred offspring in poorer health. This is clearly an epigenetic effect, Michels said, but it is not transgenerational. It results instead from exposure to a transient environmental stimulus—the food the mother mouse eats—during a critical period of fetal development. Such exposure can induce permanent changes in metabolism and susceptibility to chronic disease, but doesn’t mean that the epigenome of the mother passed to the offspring. Instead, the effect is more like the changes seen in honeybee larvae. Those fed royal jelly become queens: large, long-lived, fecund, showing low brain activity. Those fed worker jelly are short-lived, small, but much more neurologically active.
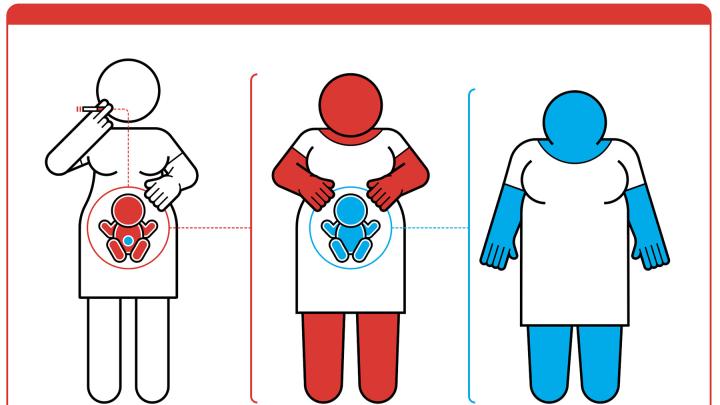
Claims that epigenetics has influenced multiple generations in families whose founders survived famines or other traumatic events are especially difficult to substantiate in humans, Michels explained: the effects would need to carry across four generations in the maternal line to prove that the effect was inherited. That is because female reproductive cells are fixed even before birth. Smoking by a pregnant mother would therefore affect the mother, her unborn child, and also (if that unborn child is female) the third generation through the reproductive cells of that unborn child (see illustration). In males, because sperm is generated continuously throughout life, only three generations are required to prove epigenetic inheritance, but even that timespan is too long for any researcher studying humans. Even in laboratory animals, Michels said, no researcher has proven transgenerational epigenetic inheritance. Despite claims to the contrary in the most distinguished scientific journals, she asserted, every experiment to date could be explained by in-utero exposures or other mechanisms.
She allowed just one exception, which occurs through a distinct and poorly understood mechanism called genomic imprinting. This transgenerational inheritance seems to be limited to genes that control growth during fetal development. In general, the male allele of genes for growth is turned on, while the female allele is turned off; this memory of which allele is turned on or off is dutifully passed across generation after generation. (For an evolutionary theory explaining this tension over the size of unborn children, see “Prenatal Competition,” September-October 2006, page 18.)
In closing, Michels emphasized that epigenetic inheritance may exist—but it has not been proven. And given the current epidemic of sedentary behaviors and obesity, she concluded, perhaps the mechanisms that strip our reproductive cells of the memory of such epigenetically modulated states from one generation to the next are “a good thing!”