Non-scientists generally think of “circadian clock” as a metaphoric term. There’s nothing literally ticking away inside the human body, helping align it to the regular cycle of day and night. But synthetic biologists from Harvard Medical School (HMS) and the Wyss Institute for Biologically Inspired Engineering have created something just that tangible: a transplantable, bioengineered 24-hour clock, which functioned by itself after being inserted into a bacterium that doesn’t typically have a circadian rhythm.
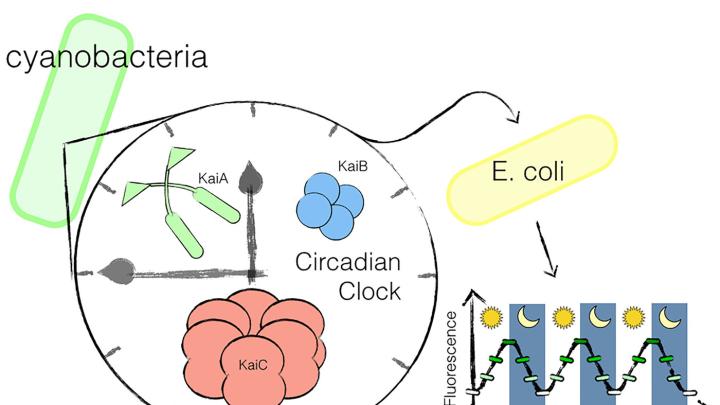
Anna H. Chen, a graduate of HMS’s systems biology department, led the new research while a doctoral student in the lab of Adams professor of biochemistry and systems biology Pamela Silver. The team, which published its work in the journal Science Advances on Friday, June 12, began by examining the mechanism behind the circadian rhythm in the cyanobacterium Synechococcus elongatus. These photosynthesizing bacteria use three key proteins to regulate their cycles of day and night. Past research had already established that, even when simply combined in a test tube, the three will bind and unbind in a regular, circadian rhythm. Chen used these proteins to build an “oscillator”—a biological function that turns off and on in a regular pattern—and transplanted it into the gut bacterium Escherichia coli.
For synthetic biologists, the process used to identify and deploy these proteins is, by now, a relatively well-understood one. The “real genius” of Chen’s experiment, Silver reflected, was therefore in its specific implementation: building a system that would put these tools together in the right way, and provide a mechanism to prove that it had worked. The team created a fluorescent protein marker that would operate “downstream” of the oscillator. Over a three-day period, they observed it turn on and off on a regular, 24-hour cycle, proving that the rhythm had taken hold in the normally non-circadian E. coli,
The result was the first transplantable, 24-hour circadian clock. While other researchers have been able to produce either a time delay or a rhythm on a shorter time-scale, the Silver lab is the first to create a system using a 24-hour cycle, which allows it to “interface with virtually all of life,” Silver said. The experiment, Chen explained, provided a proof of principle. Now, scientists can swap out the pulsing fluorescent marker protein for any one of a variety of other functions, which could then be similarly tied to a daylong cycle. “This back and forth is not just a random back and forth,” Chen added. “That 24-hour timing is really important to humans, because we live on the day-night cycle. We live on this Earth.”
This new clock is one of a number of biological building blocks that researchers in Silver’s lab have begun producing over the last few years (see, “Synthetic Biology’s New Menagerie,” September-October 2014, page 42). The burgeoning field of synthetic biology, in which her lab has played a leading role, is specifically oriented toward creating real-world uses for these newly engineered pieces of biology, and the authors note several potential applications for a transplantable circadian clock.
Chen pointed to several possible medical uses, which would take advantage of the wide role that microbiomes play in human health. Most obviously, a system built from this research could help individuals whose body clocks have fallen out of sync with their environments. It could also aid in the treatment of obesity and glucose intolerance, which researchers have linked to out-of-sync circadian rhythms in gut bacteria. These synthetically engineered body clocks could also be used to help automate the delivery of drugs at specific times of day, and to control the action of microbes used in industrial processes.
For Silver, the research also represents a step forward in one of her lab’s larger research goals. For more than a decade, she has been interested in building a biological “timer”—a cell that could count up the amount of time that had passed since it had been exposed to a certain stimulus—and the day-to-day rhythm of the circadian clock could be an important development in that project.
Silver added that she hopes that other researchers take the circadian clock tool and begin to refine it for these kinds of diverse applications. “The whole point of making these things,” she reflected, “is for other people to use them.”