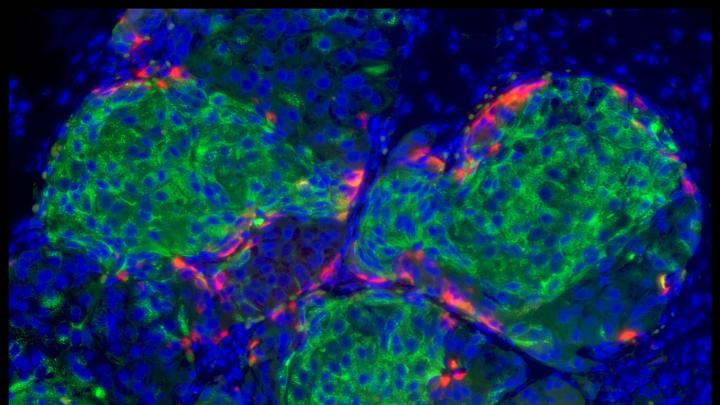
In what may lead to the biggest breakthrough in the treatment of Type 1 diabetes in three decades, Xander University Professor Douglas Melton and colleagues have figured out the complex series of steps necessary to turn stem cells into beta cells. Beta cells are the sugar-sensing, insulin-secreting cells of the pancreas that are missing in Type 1 diabetics, casualties of the body’s own immune attack on itself.
Scientists first discovered in the 1920s that insulin is the necessary substance most diabetics lack. For decades thereafter, physicians injected the protein, which at the time was purified from animals, into patients as a treatment. In 1978, the gene for human insulin was cloned, says Melton, leading in the early 1980s to what is now a major industry: the production of injectable human insulin. “While there have been continual improvements in the types of insulins—long and short-acting—fundamentally since the 1980s, there have been no advances other than providing insulin to inject into patients,” Melton explains. “This is a kind of life-support for diabetics. It doesn't cure the disease and leads to devastating and very costly complications…such as heart failure and peripheral neuropathy, and other unpleasant consequences of patients not being able to accurately control their blood sugars or their metabolism.”
“We wanted to replace insulin injections” with “nature's own solution,” says Melton, who has been a leading scientist in and advocate for the field of stem-cell biology ever since he switched from studying developmental biology in frogs after his young son, and later his daughter, were diagnosed with Type 1 diabetes.
He is now co-director of the Harvard Stem Cell Institute (HSCI) and co-chair of the Harvard department of stem cell and regenerative biology (in the Faculties of Medicine and of Arts and Sciences).
What Melton reports in the journal Cell on October 9 is that his lab, including co-first authors Felicia W. Pagliuca, Jeff Millman, and Mads Gurtler (as well as a Harvard undergraduate and others), have succeeded in developing a procedure for making hundreds of millions of pancreatic beta cells in vitro. These cells, Melton explains, “read the amount of sugar in the blood, and then secrete just the right amount of insulin in a way that is so exquisitely accurate that I don't believe it will ever be reproduced by people injecting insulin or by a pump injecting that insulin.”
The cells share key traits and markers characteristic of beta cells with those from healthy individuals, including the packaging of the insulin they secrete in granules. In diabetic mice, they cure diabetes right away, in fewer than 10 days, Melton reports.
The challenge of creating such cells was described in this magazine in 2004:
Type 1 diabetes, the problem that concerns Melton, is well-suited to stem-cell therapy. It involves a single cell type, the beta cell, that is either missing or present in numbers too low to regulate blood-sugar levels. "If you could place that cell type back into a person [so] that it was not subjected to autoimmune attack, where it could be healthy and thrive, even outside the context of the pancreas, then you could cure diabetes." One current therapy for Type 1 diabetes — the Edmonton protocol—involves injecting beta cells from three cadavers into a patient’s vein; the cells seed the liver and work from within that organ. But nonsteroidal immunosuppressants are required to control both autoimmune attacks and rejection of the foreign cells by the patient’s body. Nevertheless, a first test of beta cells created through directed differentiation of human [embryonic stem] cells might be to use them in the Edmonton protocol. The next step might be to create a device—beta-cell tissue grown on a synthetic three-dimensional biomatrix—and encapsulate it in a Gore-Tex-like membrane that would allow glucose and insulin, but not immune cells, to pass through.
But the how of creating beta cells from embryonic stem (ES) cells—directing the process of differentiation in either embryonic stem cells or induced pluripotent stem cells (derived from adult cells) to make any specific cell type, for that matter—has eluded scientists for more than a decade. Recapitulating the normal development program in a petri dish has proven extremely complicated, because a protein signal that has a certain effect at one stage of the process—guiding an ES cell to become, for example, one of the embryonic “germ layers” such as endoderm (from which the gut, liver, and pancreas develop)—might have an entirely different effect at a later stage, or in a higher concentration, or within a different environmental niche in the body.
The discovery reported today in Cell was thus not the result of serendipitous biological code-breaking, says Melton, but rather of “hard work.” “What we did to solve this problem is study all the genes that come on and go off during the normal development of a beta cell in mice and in frogs and in the human material that we could get access to. Once we knew which genes come on and go off, we then had to find a way to manipulate their activity…with inducing agents.” Melton and his team tested hundreds of combinations of small chemicals and growth factors before hitting on a six-step procedure in which two or three factors are added at each step, and in which the factor, its concentration, and the duration of its application all mattered.
The result was a scalable differentiation protocol that will be usable in industrial production of beta cells. “We are very excited about this,” Melton says, because “it provides for Type 1 diabetics, in my view, half of the solution to their problem”: victims lack beta cells, and have an immune system that attacks and kills those cells. “So problem one is replacing [beta cells], and these cells are suitable for that kind of replacement” in combination with some kind of immune protection.
Melton thanked not only the 50 or more students who have worked on this problem in his lab during the past 15 years, but also the philanthropists (including the Juvenile Diabetes Research Foundation, the Helmsley Trust, numerous donors to the Harvard Stem Cell Institute, the National Institutes of Health, the JPB Foundation, and Mike and Amy Barry) who supported it, especially during the period when government restrictions made work with human embryonic stem cells nearly impossible.
How soon could a transplant therapy protocol be ready for type 1 diabetics?
“Patients ask when these cures are coming,” Melton said in a conference call with reporters, “and none of them touch me more than my own children, who ask me that all the time. I would say this. We now know we can make these cells. We have to transfer the protocol to what is called GMP, good manufacturing practice, so that it can be compliant with the very reasonable FDA regulations. So the protocol has to be done with highly purified factors. That is likely to take us about a year. And then…we have to choose the method for Type 1 diabetes that will allow us to put the cells in the patients and protect them from an immune attack.”
While that could be achieved with immunosuppressants, Melton favors an encapsulation device and cited an “encouraging collaboration” with the lab of Daniel Anderson [Goldblith professor of applied biology at MIT’s Koch Institute for Integrative Cancer Research], who has developed a chemically modified alginate that coats and protects islets, clusters of beta cells. Melton estimates that an encapsulation device would be about the size of a credit card.
Elaine Fuchs, Lancefield professor at Rockefeller University and a Howard Hughes Medical Institute Investigator who is not involved in the work, hailed the discovery as “one of the most important advances to date in the stem-cell field, and I join the many people throughout the world in applauding my colleague for this remarkable achievement. For decades, researchers have tried to generate human pancreatic beta cells that could be cultured and passaged long term under conditions where they produce insulin. Melton and his colleagues have now overcome this hurdle and opened the door for drug discovery and transplantation therapy in diabetes.”
Jose Oberholtzer, associate professor of surgery, endocrinology and diabetes, and of bioengineering at the University of Illinois at Chicago, director of its islet and pancreas transplant program and chief of its division of transplantation, said the work described in today’s Cell “will leave a dent in the history of diabetes. Doug Melton has put in a lifetime of hard work in finding a way of generating human islet cells in vitro. He made it. This is a phenomenal accomplishment.”
 ">https://www.youtube.com/watch?v=3t8nxwMXHJ0]