After what seemed to be mild car accident five years ago, “John” began suffering from a host of symptoms—headaches, fatigue, irritability, difficulty concentrating. At the time of the accident—John was rear-ended by the driver behind him—he was diagnosed with concussion and mild whiplash. But he and his wife had been struggling with the aftermath ever since.
Brain scans showed no visible damage, and during the next few years John saw several doctors and specialists who gave him a haphazard regimen of drugs and recommendations, but no solutions. Apathy, depression, anger, and mood shifts strained his marriage and family life to the breaking point. “It was just an awful situation,” he says. Finally his wife got him an appointment with Beth Adams, a neurotrauma rehabilitation specialist and case manager at Spaulding Hospital North Shore. Adams diagnosed post-concussion syndrome and connected the couple with doctors including Charlton professor of physical medicine and rehabilitation Ross Zafonte at Spaulding Rehabilitation Hospital in Boston.
Seeing the team of specialists there “has completely changed my life around,” John says. He has been on a more systematic regimen of medications for his symptoms, and is receiving cognitive behavioral therapy to learn to manage the mood swings and fatigue.
John is one of more than five million people in the United States living with the long-term effects of a traumatic brain injury (TBI) caused by the sudden force of a fall, hit, or blast. Some injuries leave patients alive but unconscious or severely impaired. Others are seemingly mild, yet cause subtle but persistent changes in mood, memory, and cognitive abilities. An estimated 1.4 million Americans sustain a traumatic brain injury every year, and millions more suffer sports or recreation-related concussions. (Most of the latter recover quickly, but some experience symptoms for months or years.) Among U.S. soldiers who have sustained injuries in Iraq and Afghanistan, one estimate puts the rate of TBI at nearly 20 percent.
We tend to think of a traumatic injury as a simple, physical process, like a cut or bruise. But Zafonte, chair of the department of physical medicine and rehabilitation at Harvard Medical School (HMS), says it is more accurate to think of TBI as a disease, because its effects extend well beyond the physical injury and can unfold over long periods of time. Unlike the damage resulting from a stroke, which is often localized to one part of the brain, traumatic injuries often affect many areas of the brain in sometimes unpredictable ways.
TBI has attracted new interest recently, in large part reflecting growing awareness of the problems of soldiers and of such high-profile victims as Arizona Congresswoman Gabrielle Giffords and athletes who have died or developed brain disease after multiple hits to the head. Zafonte says the field was largely quiet when he arrived at HMS several years ago, but he now expects Harvard to become a leading center for work on TBI in the next few years. Today, researchers at Spaulding and other Harvard-affiliated hospitals are gathering data about patients and investigating therapies and interventions that could improve recovery from acute injuries or related long-term effects. Using imaging, animal studies, and experiments on cultured cells, they hope to help dispel the mysteries surrounding brain injury.
The Spectrum of Consciousness
Improvements in medical care have saved the lives of people with some of the most severe brain injuries. But there are consequences of success: the healthcare system now faces the long and complicated challenge of rehabilitating these patients. “Not only are all these people surviving,” says Zafonte, “but they’re surviving severely disabled.”
Joseph Giacino, director of rehabilitation neuropsychology at Spaulding and associate professor of physical medicine and rehabilitation, sees some of the most severely injured patients, those who have often been dismissed as hopeless. He was recruited to the hospital a year and a half ago to lead a program of treatment and research on disorders of consciousness—seeing patients with TBI and other conditions whose injuries have impaired their consciousness in some way.
Consciousness is not simply an on/off state, but a spectrum: at one end are patients trapped in comas in which their movement, awareness, and even respiratory systems are entirely switched off. Those who are awake but show no evidence of awareness are considered to be in a vegetative state. They often transition to what is called a minimally conscious state: awake and sometimes aware of their surroundings, but unable to respond consistently to any but the simplest questions.
This is the state that grips Anthony Adamo now. In a conference room at Spaulding, Giacino sits with physiatrist and instructor in physical medicine and rehabilitation Ronald Hirschberg and several residents and nurses to discuss Adamo’s case and assess his progress. A man in his fifties who owned a construction business and has a wife and two children, Adamo suffered a fall on the job that caused a severe brain injury. Now, three months later, he sits in a wheelchair, moves fitfully with only half of his body, and is wearing a helmet to protect his skull, which still has an opening after a brain surgery. As occurs in many patients, the events after his injury damaged his brain as much as the initial trauma. Bleeding and swelling made it necessary for surgeons to remove brain tissue. Before meeting with him, Giacino and Hirschberg reviewed MRI images of his brain that showed in stark relief a gap on one side.
Now, Giacino stands next to Adamo and attempts to communicate with him. He asks Adamo if he knows where he is, and asks him to touch his nose. The patient seems confused and mumbles answers that trail off. Giacino holds Adamo’s hand, which has been moving distractedly.
“Who’s your favorite football team?” Giacino asks. “The….”
There’s no reply. Giacino persists. “New England….”
“Patriots,” Adamo responds softly.
“Who’s their quarterback? Tom…..”
“Brady.”
For Adamo’s doctors and nurses, progress comes in these small victories. Severely impaired though he is, Adamo has improved dramatically. But he still doesn’t consistently answer yes and no questions correctly, and when Giacino gives him a plastic cup and asks him how it’s used, he doesn’t respond. Tasks like recognizing an object’s use require multiple brain areas working together and clear thinking—but at this point in his recovery, Adamo seems too distracted. When he can accomplish at least one of these two tasks, he’ll be classified in a higher state of consciousness, called the post-traumatic confusional state, in which higher functions remain impaired but basic activities of daily living reemerge.
Though this classification system is helpful, injuries like Adamo’s are very hard to categorize and track. Patients seem to get better and then lapse. Whether abilities like language are damaged by an injury or simply hampered by confusion, distraction, or incapacity to attend to questions is difficult to know.
After the examination, Giacino and Hirschberg discuss Adamo’s progress, medications, and possible therapeutic strategies. Hirschberg says it is not yet clear how far he will ultimately progress, but that he has the potential to make a meaningful—if not complete—recovery. Giacino says that while many patients in this situation have been dismissed as unsalvageable, “we now have long-term data that patients get better”—vital evidence in making the case to treat them.
Giacino has been a leader in efforts to establish standards of care for patients with disorders of consciousness. In addition to treating them, he is gathering a wealth of information about their condition and progress and has helped create a consortium of 10 centers in the United States and Europe that has amassed a large data set and will begin releasing its initial findings this year. “We have no gold standard for measuring consciousness and very little evidence concerning treatment effectiveness,” he says; part of his goal is to bring more rigorous definitions and standards to this slippery concept, to better track which interventions work.
He is also involved in studies to look for drugs that could speed recovery. Currently, he says, “There is not a single proven treatment for promoting recovery from brain injury.” Instead, physicians manage medical symptoms and rely on rehabilitation to help restore cognitive and motor functions.
Giacino has also been involved in brain-imaging studies which have, in the past few years, revealed surprising activity in patients who appear to be unconscious, suggesting that even in the case of devastating brain injury, there may be a residual capacity for the brain to function, which might be aided by therapies. He is now working to use functional MRI imaging as a window into the minds of unresponsive patients. Perhaps looking into the brain will help clinicians make better decisions about how to help them.
Comprehending Concussion
Concussions, the most common brain injuries, occur when a rapid rotational acceleration to the head—such as a hit or fall—causes a temporary loss of brain function. It doesn’t cause detectable structural damage, but its symptoms can range from headaches to loss of consciousness to seizures. New research has raised puzzling questions about these incidents.
Concussions related to sports are common among children and teenagers, and probably more widespread than records indicate. Studies have found that one-third of athletes don’t recognize their symptoms as concussion (most concussions don’t involve a loss of consciousness) and many athletes ignore or avoid reporting any symptoms they may have (see “Hits, Heads, Helmets,” January-February 2010). Nevertheless, awareness has grown: “There’s much more understanding of the severity of the injury,” says neurosurgeon and associate professor of surgery Mark Proctor, who leads the Brain Injury Center at Children’s Hospital.
Yet “concussion” is still frequently used to mean a mildly traumatic brain injury, and barely a decade ago, says pediatrician William Meehan, director of the Sports Concussion Clinic at Children’s Hospital, the idea that concussion could cause more than temporary problems was controversial. “People thought you just got better,” he explains—and because most concussions do resolve quickly, people who complained of long-term symptoms were sometimes dismissed as malingerers.
But studies of athletes who have experienced concussions show a measurable drop in cognitive function after a single incident. Most subjects recover, but as they sustain additional injuries, the declines become more pronounced, and even permanent. Meehan and colleagues decided to explore the issue in animal studies (“Mice don’t malinger,” he says). By developing a system for inducing a concussion in anesthetized mice, the scientists have been able to study the injury’s effects in much more detail than could be done in humans. The research affirms what has been seen in athletes: even when concussions don’t cause visible structural damage to the brain or individual brain cells, they can still impair performance on memory tests.
The model has also facilitated study of the effects of multiple concussions—a common experience for athletes. The investigators find that repeat injuries cause cumulative impairments, particularly if there’s little recovery time between episodes.
One puzzling aspect of concussions is why they affect people differently. Roughly 98 percent of those who suffer such an injury are symptom-free within a month. Meehan says the question is, “Is there a way to predict who will fall into that 2 percent?” Researchers seek to determine if certain people are susceptible to severe or long-term effects of concussion because of a genetic predisposition or some other factor. Using genetically altered mice, the team is testing the effect of one possible genetic connection, a variation in the gene apolipoprotein E that has been linked to a worse outcome after brain injuries. It’s not yet clear whether the same gene has a role in concussion.
Meehan hopes that these models will help answer other questions, such as whether concussion’s effect on the still-developing brains of children and adolescents differs from its impact on the brains of adults. Another concern is a rare event called second impact syndrome, which occurs when an athlete returns to play too soon after a concussion and suffers another, leading to severe and life-threatening swelling of the brain. Not surprisingly, Meehan and Proctor advocate for better awareness of the dangers of concussion, better medical attention for athletes who suffer injuries, and stricter rules about return to play and contact in school sports. They also collaborate with the Center for the Study of Traumatic Encephalopathy at Boston University, which is studying whether multiple concussions can cause chronic traumatic encephalopathy (CTE), a devastating degenerative brain disease that can cause dementia and depression.
“TBI on a Chip”
Although TBI begins with a physical trauma, it quickly leads to a series of chemical and structural changes in the brain—some immediate, others unfolding slowly over time. For Kevin “Kit” Parker, Tarr Family professor of bioengineering and applied physics in the School of Engineering and Applied Sciences, this interface between physics and chemistry represents a potential therapeutic opportunity. Parker’s research usually focuses on the heart (see “Life Sciences, Applied,” January-February 2009), but he and other researchers in his lab have begun to take on the question of TBI at a cellular level. Their interest is personal: nine lab members are military veterans (including Parker, a major in the U.S. Army), so they have seen how improvised explosive devices can devastate the lives of soldiers who survive such attacks.
During a blast, a quick pressure wave rips through the air; the question is what happens when that wave meets brain tissue. Parker says many people assumed that blast-induced TBI damages the brain by ripping small holes in brain cells, causing them to die. He had a different theory: that the mechanical forces of a blast might trigger a chemical shake-up within the cells, initiated by proteins called integrins at the cell surface. (He had previously studied these proteins in other types of cells.)
Parker first outlined this hypothesis in a 2006 conversation with Borna Dabiri ’07, now a graduate student in SEAS. During the next few years, Parker’s team at the Disease Biophysics Group at SEAS and the Wyss Institute for Biologically Inspired Engineering developed a way to study blast-like forces affecting individual cells. This past July they published papers in the Proceedings of the National Academy of Sciences and PLoS One showing that their original hunch was correct.
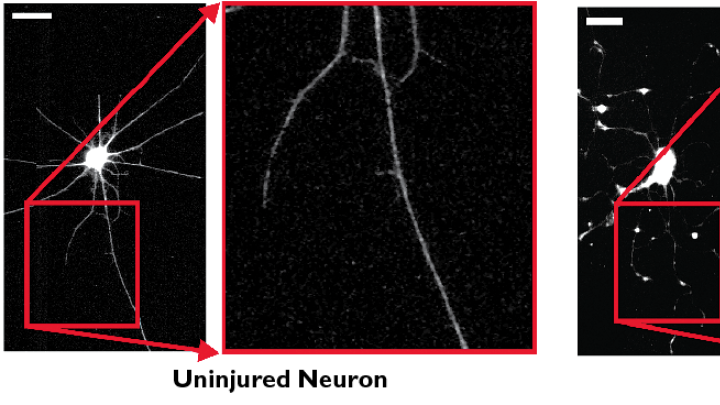
Integrins help anchor cells’ inner “skeletons” to the outer tissue in which they are embedded, but they also activate chemical changes inside cells. The studies provide evidence that integrins may translate mechanical blast forces into cellular damage in both neurons and blood-vessel tissues. The team placed rat neurons on a plastic surface and then subjected them to brief, abrupt stretching forces, using a highly controllable, piston-like device developed by graduate student Matthew Hemphill and staff engineer Josué Goss, a U.S. Marine with two combat tours in Iraq who used his knowledge of explosives to mimic the forces passing through the brain in a blast. As a result, Parker’s team found it possible to induce TBI-like injuries in cells without physically ripping them.
To study integrins in more detail, they also attached tiny magnetic beads to neurons coated with a protein that binds specifically to integrins. They found that applying an electromagnetic force to beads attached to the axons—the long tails—of neurons could injure them, and that forces stressing one bead propagated through the cell’s skeleton down another axon, causing that distant axon to break or be injured. Parker says the propagation of forces through neurons explains why physical damage in human brains may appear far from the original injury site.
The researchers used a similar technique to study mechanical forces in the cells that line blood vessels, creating artificial vessels from sheets of smooth-muscle cells. High-speed pulses of stretching, they found, caused the blood vessels to contract and the sheets to fold, inducing chemical changes in the cells. That result suggests that integrins mediate another medical phenomenon that occurs in brain injuries but is particularly common to blast victims: cerebral vasospasm, a dangerous narrowing of the brain’s blood vessels that can emerge days to weeks after the initial injury. Treating damaged neurons and blood-vessel cells with a drug that inhibits a protein activated by integrins lessened the effect of the injury. Parker hopes that by isolating a chemical process involved in the pathology of TBI, the team will find a drug that can be given to soldiers before or immediately after an injury to lessen its damage to the brain.
These cell studies are the first step in what Parker calls a “TBI on a chip,” a highly simplified model to screen for drugs to treat brain injury. He wants “to build a one-cubic-millimeter piece of brain” that he could then grow in dozens of tiny wells in microfluidic chips suitable for high-throughput screening of possible drugs. Parker’s team is now at work on growing different types of cells together, which would mimic the multiple cell types found in the brain. The chips would be subjected to forces that simulate a blast, and then screened for chemicals that prevent damage. Developing this kind of tool, he hopes, could entice pharmaceutical companies to direct resources into a search for TBI drugs.
“The Most Complicated Disease”
Zafonte calls TBI “the most complicated disease in the most complicated organ known to man.” This complexity explains why the disease has so vexed scientists and clinicians, and why so many clinical trials for treatments to improve recovery have failed. Traumatic brain injury remains a puzzle on many levels, from the events unfolding in brain cells to the complex and varied way those events play out in a human life.
Efforts to solve those puzzles vary as well, from Parker’s pared-down, engineering approach to the collection of data about real patients at Spaulding and other Harvard-affiliated hospitals. Zafonte leads a program on TBI and Neurotrauma for the Center for Integration of Medical Innovation and Technology (CIMIT), a nonprofit consortium in Boston that funds new techniques for monitoring and treating brain injury. Its research aims to understand the biological processes underlying mild and severe brain injuries, why individuals respond differently, and whether clinicians can better detect and monitor injuries through novel imaging techniques or even chemical changes in the blood.
Today, treating TBI patients is still a matter of trial and error, so Zafonte’s goal is a more scientific set of treatments. With all these efforts, he says, he hopes to offer his patients proven ways to help the brain heal: “It’s the way our colleagues went in cardiac therapies, in HIV, in all these other areas where there have been huge successes.”