Thanks in part to photographs of shrinking glaciers, news reports on the devastation caused by fierce storms, and the Nobel Committee, most Americans now accept the alarming possibility of global climate change caused by rising levels of carbon dioxide. Given the seeming improbability of reining in worldwide fossil-fuel consumption, scientists have begun proposing novel engineering solutions to the problem, like the one devised by geochemistry doctoral student Kurt Zenz House.
In a paper published last fall in Environmental Science and Technology, House and his coauthors—including McKay professor of materials science Michael Aziz and Daniel Schrag, professor of earth and planetary sciences and of environmental science and engineering, who directs Harvard’s Center for the Environment—suggest a new CO2 mitigation strategy modeled on Earth’s natural ability to absorb carbon dioxide. Rainwater and rivers collect CO2 from the atmosphere, creating a weak acid that gradually dissolves any silicate rock it washes over. This produces a carbon-bearing alkaline solution that eventually flows into the ocean, which functions as a vast “carbon sink.” Unfortunately, this natural cycle, known as “weathering,” is very slow—and the rate at which carbon moves from the atmosphere to the ocean is being overwhelmed by human CO2 emissions. (The atmosphere now holds an estimated 380 parts per million [ppm] of CO2, compared to 280 ppm in the pre-industrial age, and that figure is increasing by about 2 ppm of CO2 a year.)
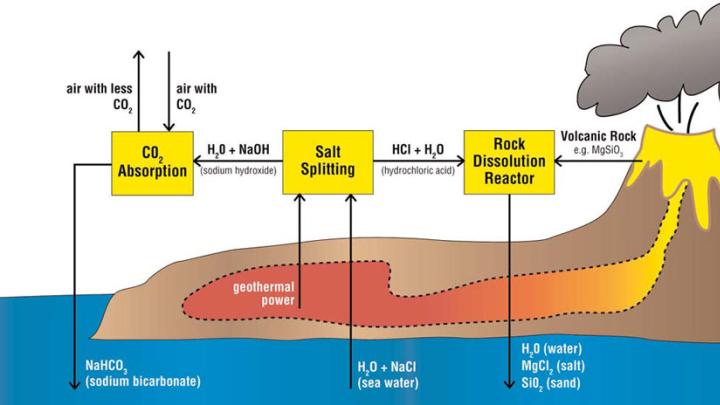
House proposes using a much stronger acid to speed up the weathering cycle and enhance the ocean’s carbon-absorbing capacity. The goal is to make the ocean more alkaline, increasing its ability to suck CO2 from the air. Such a process, eventually, could soak up an additional 3.7 billion tons of CO2 a year, about 10 percent of total annual emissions, Aziz says. In one scenario, this would involve 100 industrial plants—each comparable in water-handling capacity to a large urban sewage-treatment facility—where electric current would be used to remove hydrochloric acid from seawater. The acid would then be poured on igneous rocks, dissolving them to create a pH- neutral solution that could be returned to the ocean. To power this process, the researchers imagine building the plants at geothermally active coastal sites remote from human population centers. “We don’t want to release any CO2, because that defeats the purpose,” House explains. “Geothermal energy from volcanoes is very cheap and clean [and available], since we don’t build cities on volcanoes.” The surrounding volcanic rock also provides the right material to neutralize the hydrochloric acid.
Previous researchers considered adding alkaline material to the ocean directly to boost CO2 absorption, but a large supply of such substances is hard to find. While jogging along the Charles River in the winter of 2006, House suddenly realized that it would be simpler to remove acid from the ocean. At a conference a few months later, he discussed the idea with his brother Christopher, a geology professor at Pennsylvania State University: “I told him what I was thinking and we started doodling on napkins.” (Christopher eventually served as a coauthor of the paper.)
Part of the idea’s appeal is that it addresses all sources of CO2, not just power plants. It might also, in theory, benefit coral reefs. The rise in CO2 levels has made the oceans more acidic, a condition that harms the reefs and organisms with shells. Controlling ocean pH with this process might help.
House is used to thinking in innovative ways about the CO2 problem: he previously collaborated with his adviser, Schrag, on a plan to capture CO2 directly from power plants and bury it deep in the earth or in sediments at the bottom of the ocean. But when this latest paper appeared, and news outlets around the world reported its potential benefits, the coverage made House and his coauthors uneasy; they warn against seeing their proposal as a silver bullet for global warming. “If everything works out, this process could possibly deal with 10 percent of the carbon problem, maximum,” Aziz says. “And even that’s optimistic.”
Part of the problem is expense. It’s hard to arrive at accurate figures without further study, House says, but rough calculations suggest that the process would cost at least $100 per ton of CO2 removed, compared with $50 to $60 per ton in other CO2 sequestration techniques. Schrag is also concerned that increasing the alkalinity of seawater could trigger the precipitation of carbon as calcium carbonate; he believes this would return half the carbon dioxide to the atmosphere, making the process twice as expensive.
Another potential drawback is the possible harm to marine life created by the highly alkaline solution that would exist in seawater around the plants. “We should be very clear that we’re talking about mucking with ocean chemistry,” House says. The process mirrors a natural phenomenon, but accelerating it so drastically is “almost certain to cause some damage.” (He adds, however, that rising CO2 levels mean “we’re already mucking with ocean chemistry. We’re just doing it in a less conscious way.”) Furthermore, some of the technologies required to drive the process, including machines to electrolyze seawater, and a hydrogen-chlorine fuel cell, are insufficiently developed. Aziz believes a pilot plant could be five years off, even in the best circumstances.
Schrag, less optimistic, believes the idea is impressively clever, but faces “serious challenges that may prove insurmountable. The scale of the experiment we’re doing on the atmosphere by burning so much fossil fuel is enormous,” he says, “so trying to engineer a solution by accelerating natural processes turns out to be extremely challenging. But we have to keep thinking of ideas like this one in case there is a good solution.