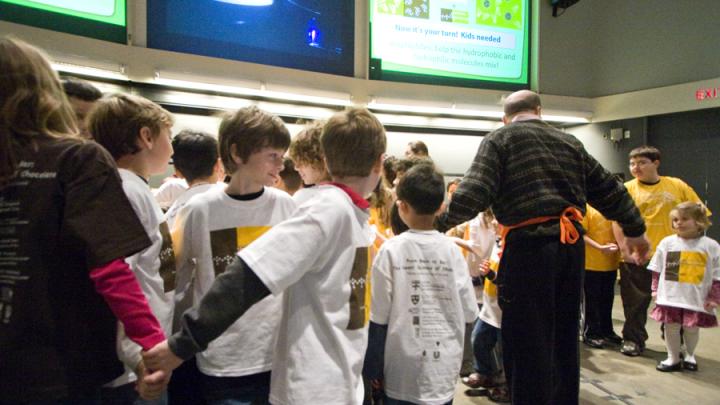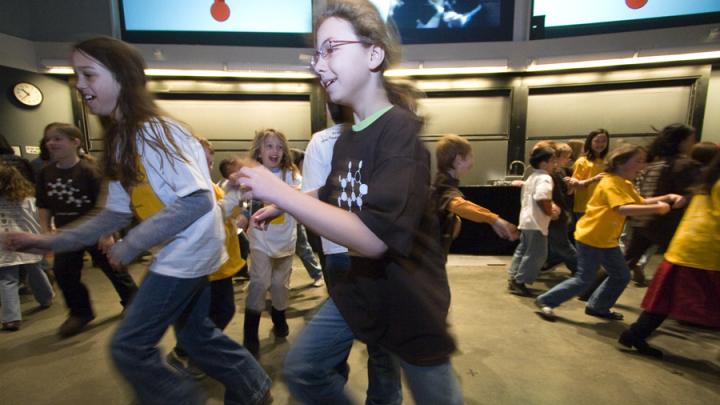In a Science Center lecture hall, Joseph professor of engineering and applied mathematics Howard Stone spoke about physics to a crowd considerably more fidgety than the students he normally teaches.
He instructed them to join him in an experiment. In unison, hundreds of envelopes rustled, hundreds of sets of teeth bit in with a small crunch (this was a tasting experiment), and then a chorus welled up: "Ewwww!"
Stone's students that morning were children and their parents, attending a free public lecture sponsored by Harvard's Materials Research Science and Engineering Center (MRSEC), among others. The substance of the experiment was a raw cocoa bean, delicious enough by some standards, but a taste apparently not yet acquired by the under-10 set.
Before a rapt audience, Stone and Amy Rowat, a postdoctoral fellow in applied physics, explained how this bitter bean becomes the sweet substance known as chocolate.
Chocolate's two main ingredients, cocoa powder and cocoa butter, don't combine easily into a smooth mixture, they pointed out. A hands-on demonstration underscored this point: one-third of the children were dressed in brown T-shirts, one-third in yellow, and one-third in white shirts with a printed design in both yellow and brown (all souvenirs handed out at the start of the lecture). As Stone gave his young volunteers instructions, the significance of the T-shirt colors became apparent: those wearing brown were cocoa solids; those in yellow, cocoa butter; and those in the multicolored shirts an emulsifier—such the soy lecithin found on the ingredient list on many chocolate labels. Following Stone's instructions, each emulsifier molecule took the hand of one cocoa-powder molecule and one cocoa-butter molecule.
For older kids and parents, the lecture offered a more sophisticated level of information: cocoa-powder molecules are hydrophilic—meaning they dissolve in water—while oily cocoa butter molecules are hydrophobic. Getting two such opposite substances to mix requires special molecules called emulsifiers or surfactants. (Here, there was a nifty demonstration with a beaker containing a layer of clear oil above a layer of blue-dyed water; adding soap, a household surfactant, and shaking turned the contents into a uniform, light-blue mixture.)
The scientists used a well-known fact—good chocolate "melts in your mouth, not in your hand"—as the entry point for a discussion of phase changes. There were test tubes of various fats (butter, Crisco, olive oil, and—of course—cocoa butter) suspended in water baths of various temperatures to show the change from solid to liquid. There was a demonstration using common household items: put "solid water"—i.e., ice—into a hot frying pan, and it quickly becomes liquid water and then gaseous water, or steam. Another tasting experiment involved placing two different types of chocolate on opposite sides of one's tongue and comparing the speed at which the samples melted (an experiment the audience found decidedly more pleasant than the earlier one). And audience members got to try out a phase change themselves. This time, the T-shirt-clad volunteers demonstrated being frozen (standing completely still); melting (beginning to walk around the stage); and vaporizing (running).
There were four tasting experiments in all, as well as a demonstration of crystal formation involving liquid nitrogen. And there was even some chocolate trivia: the first successful chocolate factory in the United States was in Boston's Dorchester section, and its owner was a Harvard graduate: James Baker, A.B. 1760.
Through MRSEC, Stone and Daniel Rosenberg ’84 (a member of the Science Center lecture demonstration team) have been offering the annual holiday science lecture for seven years. Past topics for the lecture have included paper and printing; Einstein; polymers; candles; and pizza. The food topics have a particularly strong appeal; this year, two seatings for the chocolate event—a thousand seats in all—filled almost immediately after registration opened, and the waiting list grew to 1,400. Kathryn Hollar, director of educational programs for the School of Engineering and Applied Sciences and one of the event organizers, said Harvard has applied to give the same presentation again in the spring at the MIT-sponsored Cambridge Science Festival.
Stone clearly relishes the experience, hamming it up with exaggerated facial expressions and groan-eliciting jokes during the presentation. When a tow-headed toddler wandered into the demonstration area, Stone chased her down, swooped in to lift her off her feet, and returned her to her mother, moving with the dramatic flourish of a cartoon super-hero.
This was the end of a busy week for Stone and Rowat; a few days earlier, they had hosted a visit from Ferran Adrià, the Spanish chef whose restaurant has been judged the best in the world for three years running, and whose highly technical and experimental culinary methods overlap with what happens in Harvard science labs.
But Rowat, who studies cell nuclei and the physical properties of lipids, said teaching kids about chocolate was a highlight for her, too. All too often in primary and secondary school, she said, science is taught in a dry, bland fashion. Early in her own schooling, she said, "I never realized that science is such a creative subject."
Check back later for video from this event.