An effective vaccine remains the best hope for ending the COVID-19 pandemic. As deaths and economic damage mounted worldwide this spring, every reasonable measure to seed and accelerate vaccine development was being considered. By late May, more than 170 candidates were under development—an unprecedented response in terms of scope, speed, and cooperative scientific effort to combat a virus genetically sequenced only at the beginning of January. Initial clinical trials are being expedited, and debate has begun over the ethics of “human challenge trials”—the idea, raised by Harvard epidemiologist Marc Lipsitch and others, of deliberately infecting carefully selected volunteers to speed tests of potential vaccines’ efficacy.

Mark Lipsitch
Photograph by Kent Dayton
UPDATED 6-10-2020: The Boston Globe reports that human trials of a Johnson & Johnson vaccine, developed in collaboration with Castle professor of medicine and professor of immunology Dan Barouch, will begin two months earlier than anticipated, in July.
Although no vaccine has ever been developed and approved in fewer than four years (for mumps), scientists are nevertheless cautiously optimistic that the first doses of a vaccine against SARS-CoV-2 (as the coronavirus is formally known) might be available in the United States later this year, or early next, if emergency-use authorization is granted by the Food and Drug Administration (FDA). But even if several vaccines make it through rigorous human clinical trials and are approved for general use on that extraordinarily ambitious timeline, they will initially be in short supply, given limited manufacturing capacity, adding urgency to another question with complex ethical, practical, and scientific dimensions: who should be vaccinated first?
Developing a Vaccine
The ability to develop vaccines against an unforeseen pathogen and inject them into human volunteers in 60 days, explains Barry Bloom, former dean of the Harvard T.H. Chan School of Public Health (HSPH), was an idea first advanced by the U.S. Biomedical Advanced Research and Development Authority (BARDA), which is providing substantial financial support to several large vaccine efforts worldwide. The authority, created in the aftermath of 9/11 to protect against biological, radiological, and chemical threats, including pandemic influenza, has promoted the concept of vaccine platforms that could be quickly adapted to fight any new virus. In fact, from the time of the public release of the SARS-CoV-2 sequence to the injection of a BARDA-supported vaccine “into the first volunteer’s arm…in a phase-1 safety test,” Bloom notes, took just 62 days.

Barry Bloom
Photograph courtesy of the Harvard T.H. Chan School of Public Health
But not every vaccine will make it through all phases of human clinical trials. One expert estimated that the first two COVID-19 vaccines to be tested in humans (from Oxford University and the biotech company, Moderna) each had about a 20 percent chance of proving successful through phase-3 trials, which test safety and efficacy in large numbers of people. That is one reason BARDA and other institutions are supporting multiple parallel efforts. Another is that no single manufacturer can produce the vaccine quantities needed. “What will be limiting,” Bloom predicts, is “who has the capacity to produce at a global scale billions of doses if in initial studies [a vaccine] is shown to be safe and effective.”
Evidence that a vaccine could work at all arrived on May 20, when a Science paper showed that macaques, which share 93 percent of their DNA with humans, become sick upon exposure to SARS-CoV-2, and then develop protective antibodies as they recover (see harvardmag.com/covid-immunity-20). In an experiment led by Castle professor of medicine and professor of immunology Dan Barouch, animals exposed to the virus a second time five weeks later developed no clinically observable symptoms and displayed immunologic control of the disease. These data showed that natural protection against COVID-19 exists in primates, establishing a firm scientific foundation for the global vaccine efforts. (Developing vaccines for pathogens for which there is no natural protective immunity, such as HIV, has proven very difficult). A second Science paper that day, also from the Barouch lab, shed light on the prospects that a vaccine could also prevent COVID-19.
When immunologists assess the efficacy of a possible vaccine in the early stages of development, they look for correlates of immunity that suggest how protective it might be. The ideal is sterilizing immunity, when exposure to disease results in no signs whatsoever of infection. But Barouch notes that even with the measles vaccine, generally thought to be 95 percent to 99 percent effective, “If you get exposed to the disease, you’ll probably still get infected before your immune responses kick in and eliminate the virus. It’s prevention of clinical disease and blocking of transmission that really is the goal of a vaccine.”
In testing, some vaccine candidates elicit no immune response. Most produce reactions that range from the development of partially protective antibody responses to strong, neutralizing responses in which antibody levels match or even surpass those observed in response to natural infection, and block both infection and transmission.
Such distinctions were critical for immunologists and public-health experts evaluating the earliest published results of COVID-19 vaccine trials in May. Those studies suggested the vaccines could confer protection, but might not prevent transmission of the virus. In that case, everyone on earth—7.8 billion people—would need to acquire immunity, either through infection or vaccination, to stop the spread of the disease. The hope of achieving herd immunity—which effectively stops viral transmission when perhaps 60 percent to 80 percent of the population is immune—would be dashed. That in turn would require producing billions more doses of vaccine. (Glass vials for delivering vaccines to medical personnel who administer them to patients may become the next medical-supply bottleneck.)

Sarah Fortune
Photograph courtesy of the Harvard T.H. Chan School of Public Health
Speaking to reporters on May 21, Given professor of immunology and infectious diseases Sarah Fortune, who chairs the department of immunology and infectious diseases at the HSPH, said developments to that date had been “heartening,” while sounding “some cautious notes.” Among those first candidates were the RNA vaccine from Moderna; a killed-virus vaccine from Sinovac; and an Oxford University-developed vaccine delivered by an adenovirus vector. In studies, they protected tested animals against disease in the lungs, but not all prevented “carriage in the nose or the throat,” a potential indicator of protection against transmission. Such a result is common in vaccines, said Fortune, but “sobering” with respect to COVID-19. If transmission continues after vaccination, then “to protect the population, we’re going to have to be vaccinating many, many more people.”
The best data, she said, had come from Sinovac’s inactivated virus vaccine, and from the Barouch lab, which conducted experimental tests using DNA vaccines that probed the primate immune response to the SARS-CoV-2 spike protein, which the virus uses to enter cells. The researchers, who created six vaccine variants, found that optimal protection in the lungs and upper respiratory tract of macaques was achieved with the full spike protein, which produced levels of antibodies comparable to that seen in natural infection—useful information for other gene-based vaccine development efforts, if the correlation holds for humans.
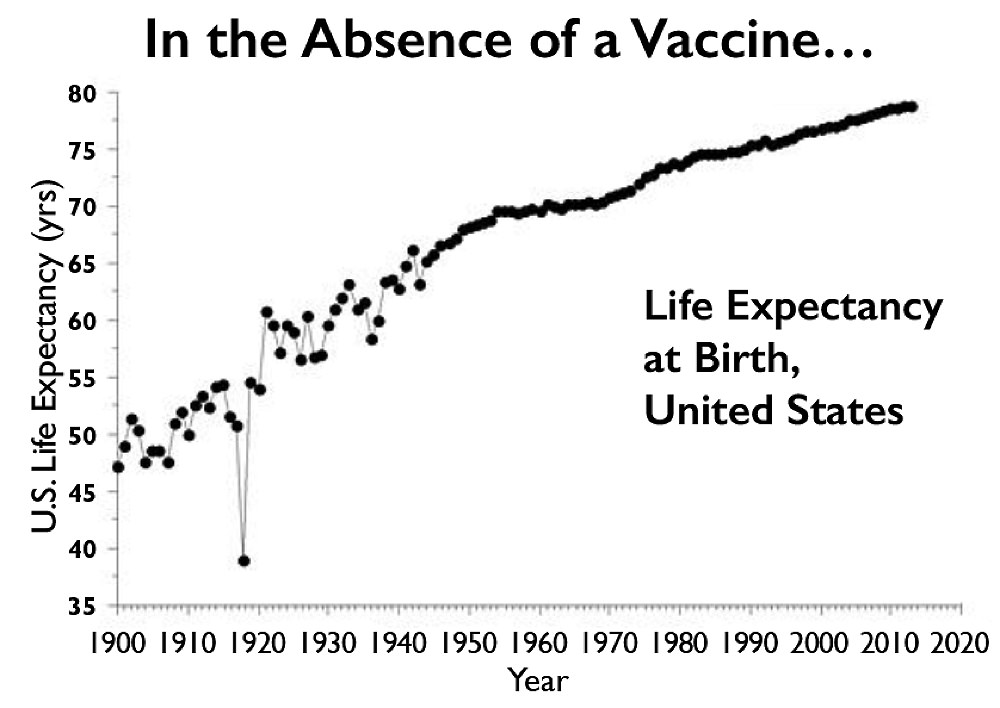
Absent a vaccine, the 1918 flu pandemic, which killed primarily adults below the age of 65, lowered life expectancy in the United States that year from 52 to 39. COVID-19 won’t have the same effect on life expectancy, but could kill just as many Americans in absolute terms.
Source: cato.org
All of the half-dozen or so vaccine platforms underlying development efforts worldwide have pros and cons. Nucleic acid vaccines made of DNA (like the six Barouch developed to test primate immune response) or RNA (like the candidate from Moderna), can be thought of as chemicals, making them relatively easy to design and distribute. They are nothing more than instructions that tell cells in the body to produce parts of the virus, training the immune system to recognize the real thing. This approach is rapid and promising, but untested at scale: no nucleic-acid vaccine has yet been approved for use in humans. Killed or inactivated virus vaccines like the one from Sinovac, have a proven track record, but manufacturing may require handling large amounts of infectious virus before it can be rendered harmless. Vaccines that use a harmless virus to deliver an antigen, like the one being developed by Oxford, have been licensed for use in humans, and have shown promise in clinical studies against numerous emerging diseases. The trick with this approach is choosing a delivery virus that the immune system has not seen before—otherwise the antigen payload may be intercepted and destroyed by the immune system before inducing a beneficial effect. Other vaccine platforms use recombinant proteins (the technology used to produce annual flu shots), or even attenuated live virus—the latter dependent on extensive safety testing, for obvious reasons.
Barouch, who directs the Beth Israel Deaconess Medical Center for Virology and Vaccine Research, has partnered with Janssen, the pharmaceutical division of Johnson & Johnson (J&J), to develop a recombinant adenovirus vector vaccine. The vaccine uses a harmless cold virus uncommon in North America, Ad26, modified not to replicate in humans, to shuttle the SARS-CoV-2 spike protein into cells, thus priming an immune response that will protect against COVID-19. Such a vaccine platform does not require an adjuvant (an additive to stimulate the immune response), Barouch explains, the way purified protein-based or inactivated virus vaccines often do, because the Ad26 viral particle is itself recognized as foreign by the immune system, and thus helps provoke an immune response.
Barouch says the development process since January has been breathtaking: just three days after the virus sequence was published, his lab had designed DNA and adenovirus vector vaccines by working nights and through the weekend. Animal studies began less than a month later, and by March, J&J, with support from BARDA, had announced that they would commit $1 billion to deliver a billion doses of vaccine, beginning with delivery of the first 300 million-plus doses starting in 2021.
The vaccine is being tested in animals, but results were not available as this magazine went to press. Barouch expects even better results than from his experimental DNA variants, based on his experience with earlier adenovirus vector vaccines he created against Zika and HIV; in those cases, he reports, the antibody responses are durable for a year or more, after only a single shot. Human clinical trials, testing both a single and booster shot (double shot) regimen against COVID-19, will begin “at latest” by September, according to a March news release—possibly sooner.
The Dilemma of Deliberate Infection
As the number of coronavirus infections passed six million globally at the end of May, and deaths approached 400,000, the desire to accelerate vaccine development even further led some scientists to actively advocate for the use of “human challenge trials” to get immediate answers on efficacy. Others, including professor of epidemiology Marc Lipsitch, director of HSPH’s Center for Communicable Disease Dynamics, said such trials should at least be considered, and not dismissed out of hand.
In the usual course of clinical testing, phase-1 and -2 trials assess safety, and whether a vaccine elicits immune responses in a small, but progressively larger, number of volunteers. Phase-3 trials recruit hundreds or thousands of volunteers representative of the total population, and then wait for them to become naturally exposed to the disease. By tracking outcomes over time, researchers can measure how effectively the vaccine protects against infection. This is the most definitive way to test a vaccine, but also the slowest. Such trials are already proceeding on an accelerated basis, with no gaps between phases.
A human challenge trial, by contrast, injects a small number of volunteers with either a vaccine or a placebo, and then deliberately infects them with the virus. Lipsitch and two colleagues explored the ethics of this approach in a paper published in The Journal of Infectious Diseases. He suggests it may be ethically superior to standard trials, because the faster a vaccine is approved, the more lives will be saved. In addition, the risk that a coronavirus vaccine could enhance the disease in some people might be detected more quickly in such a trial, preventing harm in a larger cohort. Any participants in the placebo group who became ill would receive excellent care and access to any known treatments, and only healthy young volunteers without comorbidities would be chosen, preferably from settings where they were already at high risk of natural infection. Discussions of such trials have occurred at the World Health Organization, in Congress, and at the FDA, and there is no shortage of volunteers: an independent website, 1daysooner.org, has attracted more than 26,000 people from 102 countries who wish to participate.
Jacobson Research Professor of public health Barry Bloom, the former HSPH dean, points out, however, that anyone volunteering for human experimentation must be able to provide informed consent—but not even medical experts know all the risks associated with COVID-19. Though he is not opposed to human challenge trials in principle—and he notes that this is the only substantial issue on which he and Lipsitch have ever disagreed—Bloom adds that he knows of no precedent for such trials when no approved treatment is available. And he worries about the issue of moral hazard: “In the world of economics, it occurs when someone profits, or in this case is protected, by putting others at risk. I find it morally concerning that scientific advocates are recommending live challenges…when they are unwilling to serve as volunteers themselves. That’s a dicey business.”
There are also practical objections. Bloom notes that challenge trials would be restricted to healthy young volunteers, but the high-risk demographic is “people over 60 with pre-existing conditions.” Immunity and reactions to vaccination change with age, so “You are not going to get the answers” about effectiveness in that group without testing. And in children, “The aim of any vaccine is to reduce adverse effects to one in a million or fewer.” Human challenge trials are too small to detect adverse effects, including the possibility that a vaccine might enhance disease, says Bloom. “No company would want to expose itself to legal liability because a product had not been fully vetted” by skipping large-scale trials. And by the time researchers have spent months figuring out how to infect volunteers (by aerosol or swab), and in what dose, phase-3 testing of the vaccines that are furthest along in development—expected to start this summer—will have already begun.
Lipsitch said that his aim is not to advocate for human challenge trials, but to “keep all options on the table,” since classical efficacy trials are challenging to plan: “It’s quite uncertain where there will be adequate infrastructure and incidence of infection at the time of a trial, to do it well.”
The Head of the Line
If one or more vaccines are approved for emergency use late this fall, and for general distribution beginning next year, the next question will be one of priority: who should be inoculated first?
Domestically, essential workers at high risk of exposure in sectors such as health care, public safety, the trades, the retail food industry, and transportation seem likely candidates. (Evidence from Asia showed that infections among such groups drove nearly half of early transmission.) Vulnerable populations, such as nursing-home residents, might be another high priority. But designing the best vaccination strategy to protect that population will depend on knowing how well older people tolerate the vaccine. Do they develop a protective immune response? “Vaccines sometimes work quite poorly in people over 60,” says Bloom, which might require higher doses, or special adjuvants, or different strategies, to achieve protection. Years ago, public-health experts “were quite surprised,” he says, “when they found that after vaccinating children against pneumococcus, pneumonias in grandparent-aged people almost disappeared, even though they themselves were not immunized, because the kids were not transmitting it to them.”
The question of how vaccines will be distributed also has international dimensions. Lipsitch predicts enormous “political and economic pressures on who gets access to those vaccines.” Bloom agrees. “There’ll be many who believe that because BARDA has supported vaccines, those should go to the U.S., because we paid for them. And what happens then to the rest of the world?” Such discussions, he said, should occur well in advance of the first vaccine approval.