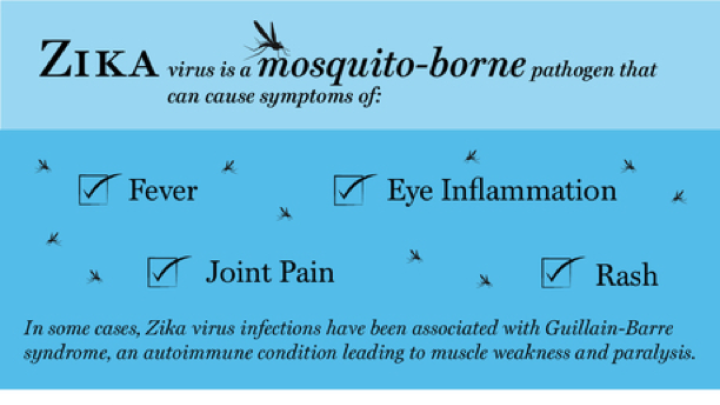
Researchers have made a surprising discovery in their search for a vaccine against Zika, a virus that can cause pregnant women to bear children with small heads (microcephaly) and other birth defects. The disease has infected more than 5,000 people from 49 U.S. states since a 2015 outbreak, mostly during travel abroad, and hundreds of thousands more in South America. Of the U.S. cases, 223—in Florida and Texas—have been attributed to local transmission from infected mosquitoes. Although rates of transmission are low now, if the virus and its mosquito carrier spread into new regions, deployment of a vaccine will be a key public-health defense against future outbreaks.
A little more than a year ago, researchers announced the promising test results of three different candidate vaccines. In mice and then rhesus monkeys, all three provided strong protection within weeks against Zika. As a result, two of those vaccines—a traditional version based on an inactivated Zika virus, and another, experimental, type based on DNA that causes cells to produce proteins present in the Zika virus in order to induce an immune response—were quickly deployed in Phase 1 human clinical trials to determine their safety and appropriate dosages (see this magazine’s earlier story “Studying Zika”).
But new tests, a year after the monkeys were initially inoculated, have revealed surprising differences in the vaccines’ efficacy. At the one-year mark, the DNA vaccine was no longer effective, reports professor of medicine Dan Barouch, director of Harvard Medical School’s Center for Virology and Vaccine Research (CVVR) at Beth Israel Deaconess Medical Center today in Science Translational Medicine (STM). The team, including CVVR’s Peter Abbink, Rafael Larocca, and other colleagues there and at the Walter Reed Army Institute of Research and at Bioqual, an animal-testing facility, did find that the vaccine based on an inactivated Zika virus (administered in two doses, four weeks apart) provided robust protection to 75 percent of rhesus monkeys after one year, a good result. But the third vaccine they had created—delivered by an adenovirus, the family that causes severe colds—proved even more effective, providing 100 percent protection to the monkeys with just a single immunization, even a year after administration. Furthermore, they were able to establish the threshold concentration of antibodies that conferred full immunity on the monkeys (though those thresholds are likely to be different in humans).
The researchers found that the level of circulating antibodies against the Zika virus was the key to all three vaccines’ effectiveness. The Zika-neutralizing antibodies were lowest in animals given the DNA vaccine, and highest in those that had received the vaccine via an adenovirus vector. “Adenovirus vector-based vaccines are typically more potent than DNA vaccines,” reports Barouch. But why that should be so, the researchers write in their paper, “remains to be determined.” Their work not only demonstrates the importance of testing vaccines for long-lasting immunity, but is also likely to be useful for development of a human Zika vaccine and, notes the editor of STM, is “instructive for vaccine development in general.”
Although adenovirus vectors take longer to produce than other kinds of vaccines, phase 1 trials of this vaccine type have now begun, and phase 2 clinical trials to test the efficacy of all three vaccines will follow. “With Zika transmission now quite low,” however, notes Barouch, “it will be very difficult to conduct traditional human efficacy trials.” This makes the regulatory path, and timeline for approval of any Zika vaccine, hard to predict. “For example, will the FDA require traditional human efficacy trials for licensure,” asks Barouch, “or will they approve Zika vaccines based on immune-response data in humans, together with animal protection data and an understanding of the correlates of protection?” Given the enhanced understanding of how these vaccines work—now possible through the work of places like Harvard’s CVVR—changes in the path to approval are possible.