The fifth floor of McKay Laboratory houses an unlikely scientific tool: small, gleaming silver, and available at department stores for a few hundred dollars. In the hands of mechanical-engineering doctoral student Emilie Dressaire, the KitchenAid mixer can whip up a fine foam full of bubbles only a few micrometers wide. More remarkable than the bubbles’ size is their resilience. Bubbles this tiny usually disappear in a matter of seconds, but a tube of this fluffy white stuff has lasted in a lab refrigerator for more than a year.
Dressaire’s bubbles could improve low-fat ice cream. They are small and sturdy enough to replace the fat droplets that give ice cream its rich texture (less persistent bubbles cause the dessert to collapse into a yogurty goop). “When people ask me what I’m doing and I say, ‘Oh, I’m working on fat-free ice cream,’ they start paying attention,” she reports. “And I can tell them more about the science and technology involved in a product of everyday life.”
Her ingredients sit beside the KitchenAid in two containers, one holding a viscous corn syrup and the other a powdery mix of sucrose ester molecules. The latter acts as a surfactant, lowering the surface tension between the liquid and the air and thereby stabilizing the bubbles. (Note that any future ice cream, though low in fat, would not be low in calories.) By mixing the ingredients together for two hours, Dressaire traps minuscule air pockets in the dense foam.
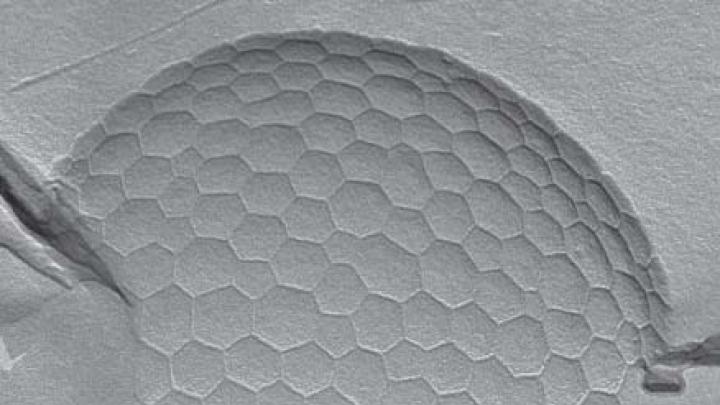
Normally, these pockets would quickly disappear, but the sucrose ester encircles the bubbles, forming a protective shell. The sucrose, or “head,” is attracted to water and sits on the bubble’s surface. The ester, or “tail,” prefers air and consequently sticks into the bubble’s center. Because the pressure inside the bubble initially is greater than the pressure outside, air slowly escapes (imagine pricking a balloon and seeing it slowly deflate). But at some point, the tough crust prevents further shrinking: the bubble “has found an optimal balance,” Dressaire explains. “The overall bubble would be happy if it could shrink more, but the shell around it doesn’t want to bend more.” Under an electron microscope, the bending shell is a landscape of dimpled pent-, hex-, and heptagons and looks like an irregular soccer ball.
Rodney Bee, a scientist at the consumer-product company Unilever, developed the mixing technique in 1997, but existing microscopes couldn’t observe the bubbles directly, leaving open the question of how the shell formed. Nearly a decade later, Joseph professor of engineering and applied mathematics Howard Stone heard Bee discuss this research at a conference and suggested that his student Dressaire could find an answer using newer technology. The two men had competing hypotheses about the bubbles’ shell: Stone suspected its polygonal surface was caused by the mechanics of the shrinking process; Bee thought that the chemical composition of the shell accounted for its geometry. Dressaire agreed to run the experiment, and Stone bought her a mixer at a local mall.
After more than two years of work, Dressaire discovered that neither the mechanical nor the chemical hypothesis alone captured the complexity of her results. “The pool of data we collected seemed to suggest that the patterns resulted from a balance of different effects that were both mechanical and chemical,” she reports. The shrinking process did determine the shapes on the surface, but that process differed depending on whether Dressaire used sucrose monoester or sucrose diester molecules. In other words, the chemical composition prescribes the mechanical process that is in turn responsible for the polygons on the surface of the shell: the chemical and mechanical explanations are, in fact, inseparable.
Dressaire’s research could prove useful beyond the market for frozen desserts. Ultrasound contrast agents, for example, already contain bubbles, and she thinks her bubbles might enable the therapy to probe deeper into smaller blood vessels. “There is a relatively broad trend of engineering simple systems,” she says. “It is particularly interesting to observe that, in a place like Harvard, where we have so many facilities, we are still interested in building simple systems.” Something to ponder over a scoop or two on the day the foam makes it from her kitchen to yours.