When it comes to our food, we are used to thinking about “good fat” and “bad fat.” Unsaturated fat (found in such foods as salmon, nuts, and olive oil) promotes health and keeps cholesterol in check; saturated fat (meat, eggs, dairy) is less healthy and should be consumed with caution; and trans fat (partially hydrogenated oils, commonly found in baked goods and restaurant frying oil, but now banned in some places) is practically poison, clogging the arteries and contributing to hypertension and heart disease.
But the body, too, has good fat and bad fat—and the difference is not one of quantity, but of kind. When most of us think of fat tissue, what we really have in mind is white fat, which stores excess calories and tends to accumulate with too much food and too little physical activity. It’s true that we need some white fat to keep us warm and to provide energy during extended periods without food, but above this minimal amount, the less we have, the better.

Courtesy of Patrick Seale and Bruce Spiegelman
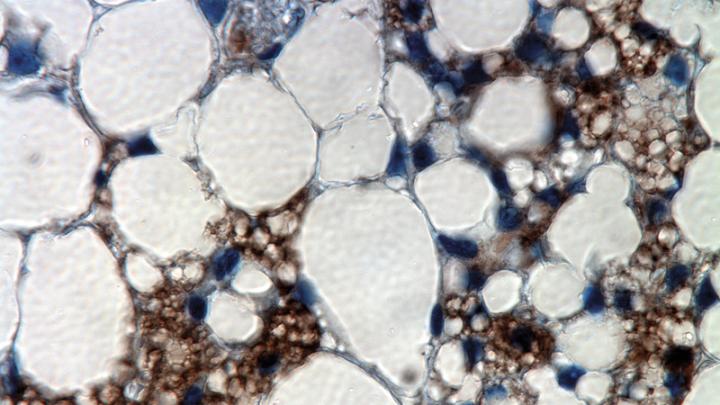
A stained culture of brown-fat cells with PRDM16 expression suppressed. Muscle fibers appear as long, stringy, reddish bodies; the green color comes from the virus introduced to suppress PRDM16; and the blue marks cell nuclei. The muscle fibers’ presence was a surprise; it indicated that brown fat is developmentally related to muscle.
Brown fat, on the other hand, is “metabolically hyperactive,” says Korsmeyer professor of cell biology and medicine Bruce Spiegelman. Instead of socking away stored energy for later use, brown-fat cells burn energy. With one of the highest rates of oxidative metabolism of any kind of cell in the body, and a very high density of mitochondria, “brown fat is the superathlete of mitochondrial biology,” says Spiegelman, who studies the wondrous tissue type. It is the sheer density of mitochondria—the cellular powerhouses that convert glucose (blood sugar) into a form of chemical energy that the body can use—that gives these cells their brown color.
Infants have a significant amount of brown fat; it generates body heat. The medical community had long recognized this thermogenic function and wished for a way to harness it, in adults, to burn off excess calories as heat—but that was considered a pipe dream, because adult humans were thought to have a negligible amount of brown fat. An accidental discovery proved otherwise.
That discovery came with the widespread use of PET scanning, a medical-imaging technique that aids the diagnosis of tumors by mapping glucose uptake in the body. Scan results show glucose uptake in the heart and brain, and in tumors, which require massive amounts of this fuel to survive and grow. But the scans also reveal multiple small glucose-uptake hotspots throughout the upper body: on top of the clavicle, in the sides of the neck, next to the vertebrae, just above the kidneys. These spots are symmetrically distributed; tumors would not be. Some scientists guessed that these mystery glucose users were brown fat deposits, a hypothesis they confirmed with experiments in humans and in mice.
It is not yet known how much variation exists among humans in the amount of brown fat we carry, and whether this has metabolic implications; in mice, at least, strains with more brown fat are resistant to diet-induced obesity.
Spiegelman’s work has focused on finding ways to encourage the growth of brown fat, and even convert other types of tissue into brown fat, and thus ramp up metabolism. Such a discovery would be a tremendous breakthrough in the treatment and prevention of obesity, which poses a health hazard in itself and contributes to many other ailments, including cardiovascular disease, arthritis, and diabetes (see “Decoding Diabetes,” November-December 2008, page 50).
In 1998, Spiegelman and assistant professor of cell biology Pere Puigserver found a protein that, when expressed in white-fat cells, made them behave more like brown-fat cells: it caused their mitochondria to “leak” energy as heat. Then, in 2007, Spiegelman and postdoctoral fellow Patrick Seale found a gene that regulated not just this mitochondrial function, but many other attributes of brown fat. Blocking expression of this gene, PRDM16, effectively converted brown fat cells into white.
All the while, everyone assumed that both types of fat cell came from a common parent, a preadipocyte, or fat-cell precursor. Last August, Spiegelman and Seale published a paper that rewrote the story. Aiming to shore up the evidence for their earlier findings, they went one step higher up the ladder and knocked out PRDM16 from mouse brown-fat precursor cells (as opposed to their earlier work with cell cultures), expecting these cells, too, to become functionally like white fat. “Instead,” says Spiegelman, “they turned into muscle in the dish. It was really a shock.” The researchers also showed that the relationship holds in the opposite direction: expressing PRDM16 in myocytes, or muscle precursor cells, caused them to turn into brown fat.
The finding has implications beyond filling in brown fat’s family tree. “If you could take out precursor cells, engineer them so they become brown fat, and put them back,” says Spiegelman, “you could presumably affect whole-body metabolic rate.” His lab is working on this already, removing cells from mice, adding PRDM16, and reinserting them. (Because the cells are autologous, this method circumvents a major obstacle to transplantation—rejection.) And his students, in collaboration with researchers at the Broad Institute of Harvard and MIT, are screening all drugs already approved for other uses by the Food and Drug Administration to see if any of them act to increase PRDM16 expression and amplify its function.
This magic pill wouldn’t be a cure-all—exercise and a nutritious diet promote health and prevent disease in ways far beyond their effect on body weight. But Spiegelman can envision a future in which a drug that acts on brown fat is used widely, in conjunction with other strategies, to treat obesity. Brown fat was first described by the Swiss naturalist Konrad Gessner in 1551 as “neither fat, nor flesh—but something in between.” He was on the right track, but it took scientists 450 years to prove it.