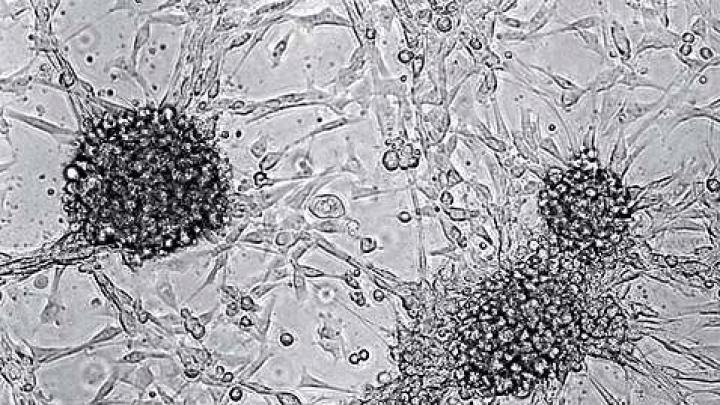
If a single word could describe the diverse disorders collectively known as cancer, it might be unpredictable. Trying to judge the course and prognosis of this meandering, unforgiving, and frequently fatal disease is almost impossible.
But Thomas Deisboeck, as director of a pioneering project to “engineer” different kinds of tumors, is trying to make cancer more predictable--and hence, more treatable. His Center for the Development of a Virtual Tumor (CViT) doesn’t grow cells in a lab or study cancer in mice. Instead, it serves as a “virtual laboratory,” using computers rather than test tubes, and three-dimensional images instead of lab animals, to foster collaboration among researchers from around the world. “At CViT, we extract data from experiments and the scientific literature, and build models with it,” explains Deisboeck, an assistant professor of radiology at Harvard Medical School. “Basing our computational models on a cell’s interaction with its environment, we can simulate everything from a single cell to an organ, and make predictions from what we observe.”
Headquartered at Massachusetts General Hospital and part of the National Cancer Institute’s (NCI) Integrative Cancer Biology Program, CViT “funds multidisciplinary research teams that take a ‘holistic’ approach toward cancer,” explains Dan Gallahan, who as deputy director of NCI’s division of cancer biology has worked closely with Deisboeck. Harvard’s contribution to the CViT project includes high-performance-computer access through the School of Engineering and Applied Sciences, and a uniquely cross-disciplinary research environment that Gallahan says provides “a critical mass of researchers across a broad spectrum of knowledge.”
Such cross-disciplinary teamwork, computing power, data availability, and better imaging have been the greatest drivers of scientific modeling, a field that has seen its most high-profile use to date in meteorology and weather forecasting. When applied to cancer research, a computer-generated model might simplify a complex metabolic pathway, revealing how millions of individual cells signal and communicate with one another: “Okay, we’ve grown enough for now,” or “We need a bit more of that hormone over here.” Carcinogens encourage tumor growth by interfering with intercellular communications, and models can show precisely how and where the interference occurs.
Deisboeck’s cancer research builds on earlier work he did as director of the Harvard-MIT Complex Biosystems Modeling Laboratory: most notably, a headline-making 2003 paper in which he asked a simple but intriguing question: Does tumor growth follow a “universal” law?
In his answer, developed with a team from Italy, Deisboeck proposed not only that a mathematical law governs cancer growth, but that it’s the same law--described in 2001 by a team of physicists and biologists in the journal Nature--that normal tissues obey. The discovery is important, he says, because it could allow physicians a better way to measure the right amount of anti-cancer therapy a patient needs at the most opportune time in the course of the disease.
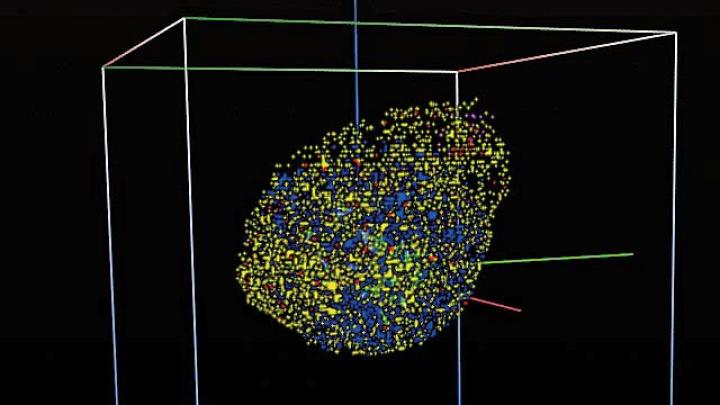
The discovery suggested that tumor modeling could work, boosting the fortunes of his nascent CViT project. The latest CViT development is a Web-based digital model repository that came online in May and provides “instant access to a variety of predictive models to scientists anywhere in the world,” Deisboeck reports. With a few keystrokes, for instance, a researcher specializing in liver cancer who wants to find out under what conditions a cell in the pancreas might become cancerous can access an online digital model created by a pancreatic-cancer specialist. The researcher can review 3-D pictures showing the cell moving incrementally from normal to diseased, and also view data providing individual snapshots of conditions as they vary over time. The models also offer a good way to follow metastasis, as a cancer in one part of the body gradually moves to another place in a sequence of steps.
Deisboeck’s tumor-modeling concepts are “quickly becoming standards in the field,” says Gallahan. “As we begin to appreciate the complexities of cancer, the approaches CViT is developing will be critical for comprehensive analysis and adaptation to a more personalized form of medicine.”