The story of smart drugs and their role in the present cancer-treatment revolution has its roots in the nineteenth century, when a strange cancer epidemic repeatedly swept through U.S. poultry farms where hens were packed tightly together. Typically the affected birds developed swollen bellies and gasped for breath. Their abdomens, when cut open, were full of masses of cellscancer. Less commonly, the birds grew large tumors on their wings. Poultry farmers were desperate: when one bird developed a tumor, the entire flock succumbed.
The mystery intrigued Peyton Rous, a physician who had accepted a full-time appointment in 1910 to direct cancer research at the newly created Rockefeller Institute of Medical Research in New York (now Rockefeller University). Rous, who had been brought up on a Texas cattle ranch, started his work after a poultry farmer brought a chicken with a wing sarcoma to his new Rockefeller lab. The researchers minced up the cancer and suspended the chopped cells in water. The mixture did not include any intact cells, yet after Rous injected the material into the wings of normal chickens, they developed sarcomas. This meant the cells had to be associated with a tumor-causing agent.
Rous next fractionated the mixture of minced tumor cells by filtering it through progressively narrow filters until the material showed nothing visible, even under a microscope. But chickens injected with the seemingly empty fluid promptly developed tumors. In 1910 Rous concluded that a virusa particle so small it could pass through any filters he hadmust cause the tumors.
 |
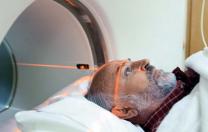
| Undergoing a PET scan: the photograph of Ken Garabadian that accompanied a New York Times article on new anticancer drugs |
| Photograph courtesy of Jodi Hilton |
That he reached this conclusion was remarkable, because the causes of cancer were virtually unknown at the time. The most relevant previous work was that of Theodor Boveri, a German biologist who had demonstrated that most cancer cells have abnormal chromosomes, the elements of heredity. Boveris findings had suggested that cancer is due to mutations of genes.
The report of Rouss experiment landed on the medical science community with a dull thud. And when Rous tried the same experiment with mouse and rat cancers, he never saw the same result. Although his work was eventually vindicatedhe shared the Nobel Prize in medicine in 1966the mechanism by which the Rous virus actually causes cancer remained unknown for many more years. Understanding that mechanism was critical to finding a way to kill off the sarcoma that might be related to it.
That effort started in the late 1940s and early 1950s, when Renatto Dulbecco and Salvador Luria, two expatriate Italian physicians who later received Nobel Prizes, initiated basic research and training programs in molecular biology at Indiana University and later at the California Institute of Technology and the Massachusetts Institute of Technology, respectively. The scientists they trained and, in turn, the students of those trainees, played key roles not only in the biology of RSV, but also in making major basic discoveries about proteins, genes, DNA, and the related structure RNA in the second half of the twentieth century. Because of that work, we know that our 20,000 to 25,000 genes are large molecules, known as polymers, that are made of DNA and lie along stretches of chromosomes in the nucleus of cells. DNA produces somewhat similar polymers known as RNA, which, in turn, engage the protein-making machinery in the cells. In that fashion, each gene produces an RNA copy, and the RNA links together amino acids, the building blocks of proteins, to produce the completed protein that the gene governs. A mutation in DNA thereby causes a mutation in its RNA and, hence, disturbance of the proteins structureits sequence of amino acids. The change in structure can alter the function of the protein and result in disease.
In the mid 1960s Howard Temin, who had been trained by Dulbecco, argued that when an RNA tumor virus enters a host cells nucleus, a viral enzyme transforms its RNA into DNA that is then incorporated into the DNA of the host cells chromosomes. The action forms new host genes that in turn produce more viral RNAs, and thereby more viral particles that leave the cell and go on to infect other cells.
Temins idea seemed preposterous: the central dogma of molecular biology had been that DNA makes RNA, which makes protein. Nobody of consequence thought RNA could produce DNA. But in the early 1970s, Temin and David Baltimore, who had also worked with Dulbecco before joining Lurias department at MIT, separately and simultaneously discovered reverse transcriptase, the viral enzyme that converts RNA to DNA. The discovery exploded the dogma, explained the life cycle of RNA tumor viruses, earned Temin and Baltimore a Nobel Prize in 1975, and led Michael Bishop and Harold Varmus to solve the mechanism by which the Rous virus causes cancer (for which they won a Nobel Prize in 1989).
Bishop and Varmus, two physician-scientists at the University of California, San Francisco, had met in California in 1970 shortly after Bishop had established a laboratory at UCSF to study tumor viruses. They showed that at some time in the distant past a benign strain of the Rous virus had invaded and incorporated its three RNA genes into the DNA of the cells of a host chicken. To do so, the virus had to copy its RNA into DNA with the reverse transcriptase enzyme and insert that DNA into the chicken cell chromosomes.
Errors can happen when reverse transcriptase does its work because many steps are involved. At least once, the reverse transcriptase forgot its role and began to copy an RNA derived from a gene that belonged to the chicken. It then copied the chicken RNA incorrectly and inserted that incorrect copy into the host chicken cells DNA. That left the cell with a DNA blueprint to fabricate a virus with four instead of three genes. The fourth, abnormal gene produced a mutated chicken protein that causes cells to proliferate wildly, resulting in cancer. Bishop and Varmus called the new cancer-causing gene src (pronounced sarc), because it was found in the mutant Rous sarcoma virus.
Bishop and Varmus coined the term oncogene (meaning a tumor-causing gene) to describe the mutant src gene and others in this new class. And they firmly established that the src oncogene arises from a perfectly normal cellular src gene they called a proto-oncogene. The idea was that certain normal genes in cells could be changed or mutated to become lethal oncogenes that produce oncoproteins, and the resulting cancers could become dependent on the oncoprotein product of oncogenes for their survival.
The Rous virus now had a basic molecular explanation, and a huge step had been taken in cancer genetics. If the protein product of a single gene could cause and maintain cancer, finding a drug that would inhibit that proteins function and cure the cancer should be possible.
By the early 1980s, several laboratories had demonstrated that the src proto-oncogene encodes a normal enzyme, a member of a large class of protein kinases called tyrosine kinases. Such enzymes transfer signals through a chain of proteins that ends within the cell nucleus. They perform their signaling function by transferring molecules of high-energy phosphorus (derived from ATP, the energy storage molecule of the cell) to tyrosine, one of their constituent amino acids, or to tyrosines in similar enzymes. Thus tyrosine kinases contribute to a network of hundreds of signaling proteins that work together to regulate cell division, normal cell death, and the functional destiny of cells. But if an oncogenic mutation of the src proto-oncogene disrupts the amino-acid sequence of the src tyrosine kinase protein, the proteinnow an oncoproteincan become hyperactive. By passing too many signals through the kinase chain to the cells nucleus, the abnormal oncoprotein causes rapid-fire cell division, diversion from the death pathway, and, hence, cancer.
Many oncogenes (such as oncogenic tyrosine kinases) have now been detected worldwide. Two of these, abl and kit, play key roles in Kens Story.
The kit oncogene was first discovered in kittens burdened by an RNA tumor virus that causes feline leukemia. Other research showed that the normal kit proto-oncogene exists in all mammalian cells, including those of humans.
 |
| The crystal structure of kit, showing how Gleevec (imatinib) blocks uncontrolled operation of the growth circuit in GIST cancer cells |
| Illustration courtesy of George Demetri; crystal structure courtesy of C. Mol, et al, J. Biol Chem 2004 |
Normal kit protein, the product of the kit proto-oncogene, turned out to be a tyrosine kinase with one important difference from the src or abl proteins. The kit enzyme is a receptor tyrosine kinase. The protein pushes its head through the cell membrane and waves it in the fluid surrounding the cell. The rest of the protein, including its signaling tyrosine, lies in the body of the cell, waiting to pass signals when an external protein latches on to and combines tightly with the waving head.
This kind of receptor tyrosine kinase is particularly useful during the maturation of a fetus. Proteins in fluids around fetal cells that bind to such receptor tyrosine kinases can cause selected populations of fetal cells to divide and differentiate, to become organs or parts of organs. Researchers have found that mice lacking essential receptor tyrosine kinases like kit or one of the specific proteins that bind to such receptors have different congenital abnormalities ranging from anemia and hair color loss to defective organs.
The understanding of kits usual role in the body started in the late nineteenth century, when the Spanish neuroanatomist Santiago Ramon y Cajal explored the neural cells of the gastrointestinal tract. Cajal, who won a Nobel Prize in 1906, wanted to know how the bowel muscle receives instructions to contract in the synchronized manner called peristalsis. The answer involved recognizing that a layer of bowel tissue contains a complex network of nerve-like cells, now named for Cajal. These large cells have multiple short extensions that protrude from their outer walls and wrap around those of neighboring Cajal cells, forming an ideal structure for passing along signals. Nevertheless, proof that these cells actually control peristalsis did not emerge until 1995, when Alan Bernstein reported that mice born without a functioning kit gene are chronically constipated and very deficient in Cajal cellsand the few they do have lack the kit protein.
Three years later, pathologists working in Sweden applied Bernsteins mouse studies to human cancer. Gastrointestinal stromal tumors (GISTs) and others like them originally had vague descriptive names because no one actually knew their cell of origin. The Swedish pathologists, suspecting Cajal cell origin, used a special stain for kit protein and found that the tumor cells stained heavily. They concluded that GISTs must arise from a cancerous Cajal cell.
One more step was necessary. A year after Ken Garabadians diagnosis, both Yukihiko Kitamura, a pathologist then at the Osaka University School of Medicine in Japan, and a team that included Marcia Lux, a Harvard medical student, and Jonathan Fletcher, a pathologist at the Brigham and Womens Hospital in Boston, reported that malignant Cajal cells in GISTs are loaded with excessive kit activity, and at least one of the two kit genes in the tumor is mutated.
 |  |
| July 2000: PET scan reveals active metastatic GIST throughout Ken's abdomen and liver. (The brain and bladder activity are normal.) | January 2001: After Gleevec therapy, no tumor metabolic activity is evident in the scan; only normal heart and kidney activity is noted. |
| PET scans courtesy of Drs. Annick Van den Abbeele, Leonid Syrkin, and George Demetri | |
Although the amount of kit protein in GIST cells is normal, its activity is enormous. GIST most often comes about when one of the millions of Cajal cells in the bowel suffers a mutation in one kit gene so that it produces an oncoprotein that stays active continuously, passing signals to the cells nucleus telling it to divide. Overwhelmed, the nucleus divides and replicates rapidly, as do its daughter cells and those of future generations. The signals also enhance the cells survival by instructing them to avoid the death pathway. A large tumor forms. That is just what happened to Ken. Then it was up to doctors like Dana-Farber Cancer Institutes George Demetri to find a treatment to kill such tumors in their patients, if they could.
The trail of discovery of one killer smart drug for patients with GIST began in 1960. At the University of Pennsylvania, geneticists Peter Nowell and David Hungerford adopted a new method for examining the chromosomes of cancer cells that had been induced to grow in a culture dish. They looked down their microscopes at the 22 pairs of non-sex-determining chromosomes of the blood cells of patients with chronic myelogenous leukemia (CML), and saw something remarkable. The pairs were normal except that, in every leukemic cell of every patient, one of the pair of chromosome 22s was even shorter than its small partner. For Nowell and Hungerford, the appearance of the chromosomes, particularly the easily discernible small 22 that became known as the Philadelphia chromosome, provided an important diagnostic test for CML.
Thirteen years later, Janet Rowley, a geneticist at the University of Chicago, looked even more carefully at the blood cells of CML patients and noticed that one of the pair of the larger chromosome 9s seemed longer than its partner. Within the next three decades, other scientists confirmed that the Philadelphia chromosome and the slightly longer chromosome 9 are due to breaks near the middle of chromosome 22 and at the tip of chromosome 9. A large fraction of one of the 22s is transferred to the tip of a chromosome 9 in exchange for a small hunk of the tip of the chromosome 9. Such exchanges are called reciprocal translocations.
 |
| June 2002: Gleevec therapy has controlled the cancer for more than two years. |
 |
| November 7: Multiple GIST metastases show increased activity, despite continuing Gleevec therapy at a higher dose. |
 |
| November 18: Multi-targeted therapy with a new drug, sunitinib, regains control of the cancer, temporarily. |
| PET scans courtesy of Drs. Annick Van den Abbeele, Leonid Syrkin, and George Demetri |
Reciprocal translocations probably occur frequently in dividing cells. After all, every time a cell divides, 22 pairs of chromosomes and two sex-determining chromosomes line themselves up, duplicate, and dump themselves properly in the nuclei of dividing cells. There have to be occasional errors in such a complex process. Fortunately, the cells that bear such errors usually die. But some translocations, such as that which causes the Philadelphia chromosome, favor a cell, and in the case of CML, the progeny of such survival-advantaged cells appropriate the bone marrow.
In the tip of chromosome 9 that is transferred to chromosome 22 is the normal tyrosine kinase gene c-abl. It produces one of the more than 500 kinases that normally work quietly together to regulate the growth of cells. CML is due to a single event in one bone marrow cell. In that cell, an innocent abl gene, yanked from its normal resting place on chromosome 9, is plastered onto the remaining bit of a broken chromosome 22 at a DNA site called bcr (for breakpoint cluster region). The forced union of bcr DNA with abl DNA on the Philadelphia chromosome forms an abnormal oncogene that produces a new and much longer fusion protein called bcr-abl. The latter forces the abl protein to signal continuously and stimulate cell growth. The result is chronic myelogenous leukemia. George Daley, then a young medical student in David Baltimores laboratory at MIT, showed in 1990 that bcr-abl can cause leukemia by itself, just as activated kit can itself force a Cajal cell to form a gastrointestinal stromal tumor. Researchers have also found that many types of cancer develop from DNA mutations of other growth-promoting or death-pathway controlling genes. Modern cancer genetics thus has grown out of one simple observation made by three investigators peering down a microscope at the blood cell chromosomes in a rare leukemia.
An effective treatment for Kens cancer emerged from an initial attack on the bcr-abl oncoprotein. Alex Matter, then a science leader at Ciba-Geigy Pharmaceuticals in Switzerland, decided to launch a major research effort in the 1980s to find drugs that would inhibit tyrosine kinases that might be responsible for human cancers. For this purpose he required a cell line expressing a tyrosine kinase and an antibody that could detect the binding of phosphorus to tyrosine and hence activation of the kinase. He received both from Charles Stiles and Thomas Roberts at Dana-Farber Cancer Institute. Thus armed, Matters team screened thousands of small molecules. They had a haystack of small molecules in which they would have to find one or two needlesdrugs that would be readily absorbed in the gastrointestinal tract, penetrate cell membranes, block the access of ATP to the target tyrosine kinases, have very low toxicity, and be reasonably specific for the target tyrosine kinase. Incredibly, they discovered three compounds that seemed to work and reported on them in 1995. One of them, STI-571 (Signal Transduction Inhibitor-571), was particularly effective. It was soon named Gleevec (imatinib).
The next question was more complicated: what should Ciba Geigy do with the drug? Serendipity came to the rescue.
Brian Druker, a research fellow in the late 1980s in the Dana-Farber laboratory headed by Tom Roberts, had taken care of patients with chronic myelogenous leukemia and knew it was caused by a translocation that mutates the abl tyrosine kinase and makes it hyperactive. When he learned of STI-571, he wondered if the compound could also inhibit bcr-abl and thereby attack CML cells. He set about to convince Matter to develop STI-571 to treat CML.
Persuading Matter was relatively easy, but his superiors at Ciba (which became Novartis after a merger in 1996) couldnt see how all the expensive research could translate into a drug effective in cancer, especially for a relatively uncommon cancer. (CML afflicts perhaps 20,000 patients per year in the United States.) At the time, no clear evidence existed that overactive tyrosine kinases caused any of the major common cancers in humans. The development of a new drug is hugely expensive. Millions must be spent on toxicity trials in animals and in toxicity and early efficacy trials in peopleand most drugs fail. Ciba could do far better by creating something for big-market problems such as coronary narrowing, pimples, hair loss, or limp erections.
But Druker, now at Oregon Health Sciences University, persisted. In 1996 Matter finally gave him a small supply of STI-571 for lab studiesand it killed CML cells. Druker implored Matter to persuade Novartis to make enough of the drug for a phase-1 clinical trial. The trial, reported in 2001, proved hugely successful. CML patients went into remission with little or no toxicitya magic bullet seemed to have arrived.
In 1999, George Demetri learned from Druker that Gleevec also shut down kit. Demetri immediately arranged a collaboration to determine whether Gleevec would kill GIST cells in a culture dish. The answer was strongly positive. Demetri still recounts the story with excitement. I cant imagine a kinder or gentler way of killing cancer cells without injuring a patient. Why kill normal cells and hope that you happen to have a lot of the cancer cells in your field of treatment? Why pummel the patient with toxic chemotherapy? Why not just give a drug that helps the body get rid of mutated cells? I wanted to start a trial of Gleevec immediately in gastrointestinal stromal tumors.
Demetri began a campaign to persuade Novartis to provide Gleevec for the treatment of GIST. A patient in Finland treated with Gleevec had shown remarkable improvement: a positron emission tomography (PET) scan utilizing radioactive sugar before and after one month of treatment showed that the tumors avidly consumed sugar before treatment but not afterward. The tumors were therefore dying. Subsequent CT scans showed that they were shrinking. Nobody had ever seen anything like this, exulted Demetri, and she had no side effects of any note.
The central management of Novartis was also impressed. They made Gleevec available to Demetri for what was to be a small clinical trial but turned out to be quite large: GIST is no less common than childhood leukemia, with perhaps 5,000 new cases a year in the United States. Scores of patients demanded access to the trial.
In the course of the trial, a second remarkable finding emerged: one dose of Gleevec could kill the cancer cells in just one day. That showed how dependent on kit GISTs can be. They may be wildly aggressive and unstoppable by carpet-bombing chemotherapy, but their Achilles heel is their utter dependence on kit for survival. No one had ever seen a solid tumor stopped in its tracks by a single dose of any therapy. The case offered proof that a concerted search for the pathways adopted by cancer cells to survive and a further search for smart drugs to block those pathways could be highly productive. It would lead to a sea change in cancer therapy.
The majority of the patients tested responded to Gleevec, although some were slower to respond than others. Eventually Demetri and his colleagues determined that the location of the mutation in the DNA sequence of a GIST-associated kit gene strongly influenced the quality and durability of response to the drug.
The most remarkable fact was that the treatment was only minimally toxic. Mild fluid retention, stomach distress, and some reduction in blood cell counts were the usual side effects. The complications of the treatment were acceptable because normal cells do not absolutely depend on kit for their survival: they enjoy a more complex interaction of signaling proteins that govern their growth. Only GISTs absolutely require mutant kit.
But there was a serious downside. Single-drug therapy of cancer is almost always associated with the development of a resistant population of cancer cells that finds a way to avoid the action of the drug, in this case by undergoing further mutations in the abl or kit molecules that prevent the drug from gaining access to them. CML and GIST cells inexorably become resistant to Gleevec.
The resistance to Gleevec by GIST cells has proven particularly devilish. The pocket or pouch in the kit tyrosine kinase molecule in which the drug sits and blocks access of ATP to tyrosine is lined by amino acids, the building blocks of proteins. If one or more of them is changed by further mutation, the drug may no longer fit in the pocket and therefore fails to function.
A pocket amino-acid mutation may occur secondarily to the pressure of the drug itself. But Charles Sawyers, an investigator at UCLA, has evidence that a very rare population of CML cells may contain a pocket mutation even before Gleevec treatment begins. Such cells become the dominant population when the sensitive CML cells are killed. That may well happen in GIST.
On the brighter side, investigators have fashioned other smart drugs to fit the mutated pocket or otherwise prevent resistance. A second approach is to add standard chemotherapy to the smart drug. Even if the carpet bombers do not work alone, they may be synergistic when added to Gleevec. Finally, researchers can take advantage of the fact that the kit signal passes through many relay stations on its way to the nucleus. Each relay station is governed by a signaling enzyme (often a kinase) produced by an independent gene. Drugs can be made that would attack the relay proteins, thereby targeting several key steps simultaneously in a signaling cascade that starts with kit and ends with growth and anti-death signals in the cell nucleus. Accordingly, in 2003 George Demetri started trying to combat drug resistance by working with new combinations of smart drugs. Given enough time, he thought he would find the right formula, and in Ken Garabadian he had found a patient eager to do his part in the fight.
Sadly, none of the several drugs that have already been designed to combat resistance worked for Ken, as they have for other patients. But we are only at the onset of the cancer-treatment revolution. The pipeline of drugs is just starting to flow. To paraphrase Churchill, We are not at the beginning of the end, but we are at the end of the beginning. Given time and determination, physicians like George Demetri will see the fruits of their labors and patients like Ken will enjoy many more years with their families.